Stability Enhancement of Freeze-Dried Gelatin/Alginate Coacervates for bFGF Delivery
- PMID: 36559042
- PMCID: PMC9781200
- DOI: 10.3390/pharmaceutics14122548
Stability Enhancement of Freeze-Dried Gelatin/Alginate Coacervates for bFGF Delivery
Abstract
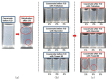
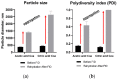
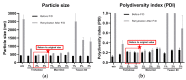
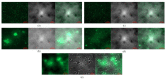
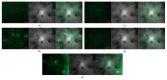
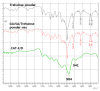
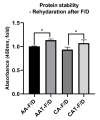
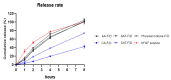
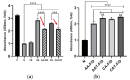
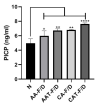
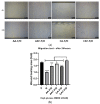
Chronic wound sites have elevated levels of proteolytic enzymes that negate the activity of topically applied growth factors. bFGF encapsulated in gelatin/alginate coacervates was protected from protease and showed better activity than bFGF in solution; however, its activity decreased with particle size and PDI increase after freeze-drying and rehydration. In this study, we aim to improve the stability of bFGF coacervates during freeze-drying to enable a topically applied growth factor delivery system for diabetic foot ulcer. Trehalose, mannitol, and Tween 80 at various concentrations were tested as cryoprotectant candidates. Trehalose improved the mechanical property of freeze-dried coacervates and physical properties after rehydration, resulting in stable size and PDI values. It also enhanced the bFGF activity in hyperglycemic human dermal fibroblasts with better cell viability, migration, and procollagen synthesis compared to the coacervates without trehalose. Hydrogen bonding interactions between trehalose and polymers probed by ATR-FTIR contribute to the stability of coacervates during freeze-drying. In conclusion, the freeze-dried gelatin/alginate coacervates encapsulating bFGF was effectively stabilized with trehalose, and the resulting coacervate composition is suggested as a potential therapeutic modality for chronic wounds including diabetic foot ulcer.
Keywords: coacervates; cryoprotectants; diabetic foot ulcer; fibroblast growth factor; freeze-drying.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Gelatin-Alginate Coacervates Optimized by DOE to Improve Delivery of bFGF for Wound Healing.Pharmaceutics. 2021 Dec 7;13(12):2112. doi: 10.3390/pharmaceutics13122112. Pharmaceutics. 2021. PMID: 34959393 Free PMC article.
-
Improved Diabetic Wound Healing by EGF Encapsulation in Gelatin-Alginate Coacervates.Pharmaceutics. 2020 Apr 8;12(4):334. doi: 10.3390/pharmaceutics12040334. Pharmaceutics. 2020. PMID: 32276508 Free PMC article.
-
Gelatin-Alginate Complexes for EGF Encapsulation: Effects of H-Bonding and Electrostatic Interactions.Pharmaceutics. 2019 Oct 14;11(10):530. doi: 10.3390/pharmaceutics11100530. Pharmaceutics. 2019. PMID: 31614977 Free PMC article.
-
Gelatin-alginate coacervates for circumventing proteolysis and probing intermolecular interactions by SPR.Int J Biol Macromol. 2018 Oct 1;117:427-434. doi: 10.1016/j.ijbiomac.2018.05.093. Epub 2018 Jun 1. Int J Biol Macromol. 2018. PMID: 29775708
-
Enhanced Oxidative Stability and Bioaccessibility of Sour Cherry Kernel Byproducts Encapsulated by Complex Coacervates with Different Wall Matrixes by Spray- and Freeze-Drying.ACS Omega. 2023 Jun 23;8(26):23782-23790. doi: 10.1021/acsomega.3c02128. eCollection 2023 Jul 4. ACS Omega. 2023. PMID: 37426239 Free PMC article.
Cited by
-
The transformation of multifunctional bio-patch to hydrogel on skin wounds for efficient scarless wound healing.Mater Today Bio. 2023 Dec 9;24:100901. doi: 10.1016/j.mtbio.2023.100901. eCollection 2024 Feb. Mater Today Bio. 2023. PMID: 38188643 Free PMC article.
References
-
- Shi X., Jiang L., Zhao X., Chen B., Shi W., Cao Y., Chen Y., Li X., He Y., Li C., et al. Adipose-Derived Stromal Cell-Sheets Sandwiched, Book-Shaped Acellular Dermal Matrix Capable of Sustained Release of Basic Fibroblast Growth Factor Promote Diabetic Wound Healing. Front. Cell Dev. Biol. 2021;9:646967. doi: 10.3389/fcell.2021.646967. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

