Exploring the Potential of High-Molar-Activity Samarium-153 for Targeted Radionuclide Therapy with [153Sm]Sm-DOTA-TATE
- PMID: 36559060
- PMCID: PMC9785812
- DOI: 10.3390/pharmaceutics14122566
Exploring the Potential of High-Molar-Activity Samarium-153 for Targeted Radionuclide Therapy with [153Sm]Sm-DOTA-TATE
Abstract
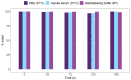
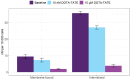
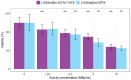
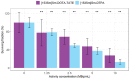
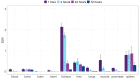
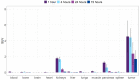
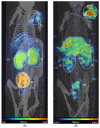
Samarium-153 is a promising theranostic radionuclide, but low molar activities (Am) resulting from its current production route render it unsuitable for targeted radionuclide therapy (TRNT). Recent efforts combining neutron activation of 152Sm in the SCK CEN BR2 reactor with mass separation at CERN/MEDICIS yielded high-Am 153Sm. In this proof-of-concept study, we further evaluated the potential of high-Am 153Sm for TRNT by radiolabeling to DOTA-TATE, a well-established carrier molecule binding the somatostatin receptor 2 (SSTR2) that is highly expressed in gastroenteropancreatic neuroendocrine tumors. DOTA-TATE was labeled with 153Sm and remained stable up to 7 days in relevant media. The binding specificity and high internalization rate were validated on SSTR2-expressing CA20948 cells. In vitro biological evaluation showed that [153Sm]Sm-DOTA-TATE was able to reduce CA20948 cell viability and clonogenic potential in an activity-dependent manner. Biodistribution studies in healthy and CA20948 xenografted mice revealed that [153Sm]Sm-DOTA-TATE was rapidly cleared and profound tumor uptake and retention was observed whilst these were limited in normal tissues. This proof-of-concept study showed the potential of mass-separated 153Sm for TRNT and could open doors towards wider applications of mass separation in medical isotope production.
Keywords: DOTA-TATE; SSTR2; samarium-153; targeted radionuclide therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Uusijärvi H., Bernhardt P., Rösch F., Maecke H.R., Forssell-Aronsson E. Electron-and Positron-Emitting Radiolanthanides for Therapy: Aspects of Dosimetry and Production. J. Nucl. Med. 2006;47:807–814. - PubMed
-
- Van de Voorde M., Van Hecke K., Cardinaels T., Binnemans K. Radiochemical Processing of Nuclear-Reactor-Produced Radiolanthanides for Medical Applications. Coord. Chem. Rev. 2019;382:103–125. doi: 10.1016/j.ccr.2018.11.007. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

