Alternative Excipients for Protein Stabilization in Protein Therapeutics: Overcoming the Limitations of Polysorbates
- PMID: 36559072
- PMCID: PMC9781097
- DOI: 10.3390/pharmaceutics14122575
Alternative Excipients for Protein Stabilization in Protein Therapeutics: Overcoming the Limitations of Polysorbates
Abstract
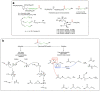
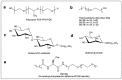
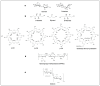
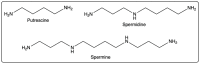
Given their safety and efficiency in protecting protein integrity, polysorbates (PSs) have been the most widely used excipients for the stabilization of protein therapeutics for years. In recent decades, however, there have been numerous reports about visible or sub-visible particles in PS-containing biotherapeutic products, which is a major quality concern for parenteral drugs. Alternative excipients that are safe for parenteral administration, efficient in protecting different protein drugs against various stress conditions, effective in protein stabilization in high-concentrated liquid formulations, stable under the storage conditions for the duration of the product's shelf-life, and compatible with other formulation components and the primary packaging are highly sought after. The aim of this paper is to review potential alternative excipients from different families, including surfactants, carbohydrate- and amino acid-based excipients, synthetic amphiphilic polymers, and ionic liquids that enable protein stabilization. For each category, important characteristics such as the ability to stabilize proteins against thermal and mechanical stresses, current knowledge related to the safety profile for parenteral administration, potential interactions with other formulation components, and primary packaging are debated. Based on the provided information and the detailed discussion thereof, this paper may pave the way for the identification or development of efficient excipients for biotherapeutic protein stabilization.
Keywords: excipient; polysorbate alternatives; protein biotherapeutic formulations; protein stabilization; surfactant.
Conflict of interest statement
The authors declare no conflict of interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
References
-
- Center for Biologics Evaluation and Research (CBER) What Are “Biologics” Questions and Answers. FDA, Center for Biologics Evaluation and Research; Silver Spring, MA, USA: 2019.
-
- Tous G.I., Wei Z., Feng J., Bilbulian S., Bowen S., Smith J., Strouse R., McGeehan P., Casas-Finet J., Schenerman M.A. Characterization of a Novel Modification to Monoclonal Antibodies: Thioether Cross-Link of Heavy and Light Chains. Anal. Chem. 2005;77:2675–2682. doi: 10.1021/ac0500582. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

