In Situ Co-Amorphization of Olanzapine in the Matrix and on the Coat of Pellets
- PMID: 36559080
- PMCID: PMC9783598
- DOI: 10.3390/pharmaceutics14122587
In Situ Co-Amorphization of Olanzapine in the Matrix and on the Coat of Pellets
Abstract
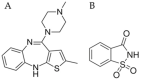
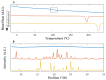
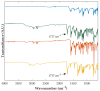
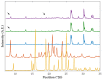
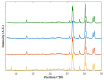
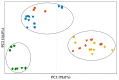
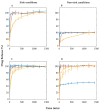
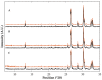
In situ amorphization is a promising approach, considered in the present work, to enhance the solubility and dissolution rate of olanzapine, while minimizing the exposure of the amorphous material to the stress conditions applied during conventional processing. The production of pellets by extrusion/spheronization and the coating of inert beads were investigated as novel methods to promote the co-amorphization of olanzapine, a poorly water-soluble drug, and saccharin. Samples were characterized using differential scanning calorimetry, X-ray powder diffraction, Fourier-transform infrared spectroscopy and scanning electron microscopy, and dissolution and stability testing. The co-amorphous produced were compared with crystalline olanzapine, or physical mixture of olanzapine and saccharin. Results suggested that the addition of water to mixtures containing olanzapine and saccharin during the production of pellets, and the coating of inert beads, induced the in situ co-amorphization of these substances. The coating of inert beads enhanced the solubility and dissolution rate of olanzapine, especially when compared to pellets coated with the crystalline drug, but also with pellets containing the co-amorphous entity in the matrix of beads. Nine months stability tests (23 °C/60% RH) confirmed the preservation of the solid-state properties of the co-amorphous form on/in pellets. Overall, results highlighted the feasibility and benefits of in situ co-amorphization, either when the drug was entrapped in the pellets matrix, or preferentially applied directly on the surface of pellets.
Keywords: (co-)amorphous; dissolution testing; in situ (co-)amorphization; olanzapine; pellets; solubility; stability.
Conflict of interest statement
Authors declared no competing interest.
Figures

References
-
- Liu J., Grohganz H., Löbmann K., Rades T., Hempel N.-J.J. Co-Amorphous Drug Formulations in Numbers: Recent Advances in Co-Amorphous Drug Formulations with Focus on Co-Formability, Molar Ratio, Preparation Methods, Physical Stability, In Vitro and In Vivo Performance, and New Formulation Strategies. Pharmaceutics. 2021;13:389. doi: 10.3390/pharmaceutics13030389. - DOI - PMC - PubMed
-
- Chen X., Li D., Zhang H., Duan Y., Huang Y. Co-amorphous systems of sinomenine with nonsteroidal anti-inflammatory drugs: A strategy for solubility improvement, sustained release, and drug combination therapy against rheumatoid arthritis. Int. J. Pharm. 2021;606:120894. doi: 10.1016/j.ijpharm.2021.120894. - DOI - PubMed
-
- Karimi-Jafari M., Padrela L., Walker G.M., Croker D.M. Creating cocrystals: A review of pharmaceutical cocrystal preparation routes and applications. Cryst. Growth Des. 2018;18:6370–6387. doi: 10.1021/acs.cgd.8b00933. - DOI
-
- Wilson V.R., Lou X., Osterling D.J., Stolarik D.A.F., Jenkins G.J., Nichols B.L.B., Dong Y., Edgar K.J., Zhang G.G.Z., Taylor L.S. Amorphous solid dispersions of enzalutamide and novel polysaccharide derivatives: Investigation of relationships between polymer structure and performance. Sci. Rep. 2020;10:18535. doi: 10.1038/s41598-020-75077-7. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

