An In Vitro Model to Investigate the Potential of Solid Dispersions to Form Pharmacobezoars
- PMID: 36559103
- PMCID: PMC9785156
- DOI: 10.3390/pharmaceutics14122608
An In Vitro Model to Investigate the Potential of Solid Dispersions to Form Pharmacobezoars
Abstract
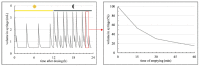
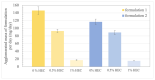
The formation of pharmacobezoars from suspensions of spray-dried amorphous solid dispersions (SD-ASDs) of new chemical entities (NCEs) and hydroxypropyl methylcellulose acetate succinate (HPMC-AS) represents a non-compound related adverse effect in preclinical oral toxicity studies in rodents. Whereas the contribution of the insolubility of the carrier polymer to this process taking place in the acidic environment of the rodent stomach is conclusive, unawareness of the extent of in vivo pharmacobezoar formation is adverse. In order to evaluate the risk of pharmacobezoar formation before in vivo administration, we subsequently introduce an in vitro model to assess the agglomeration potential of solid dispersions. To verify that the pharmacobezoar formation potential can be assessed based on the observed agglomeration potential, we conducted a sequence of experiments with two HPMC-AS-based SD-ASD formulations. In vitro, we found their different in vivo pharmacobezoar formation potential reflected by a significantly increased agglomerated mass of formulation 1 per day compared to formulation 2. In order to find an approach to reduce the agglomeration potential of solid dispersion from suspensions, we further applied the model to investigate the impact of the viscosity of the vehicle used to prepare suspensions on agglomerate formation.
Keywords: in vitro model; pharmacobezoars; preclinical testing; rodent stomach; spray dried amorphous solid dispersions.
Conflict of interest statement
Kerstin Schaefer, Thomas Nolte, Georg Boeck, Ann-Cathrin Willmann, Verena Bialetzki and Teresa Pfrommer are at the time of submission employees at Boehringer Ingelheim Pharma GmbH & Co. KG. The authors declare no conflict of interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures



Similar articles
-
Evaluation of Pharmacobezoar Formation from Suspensions of Spray-Dried Amorphous Solid Dispersions: An MRI Study in Rats.Pharmaceutics. 2023 Mar 9;15(3):887. doi: 10.3390/pharmaceutics15030887. Pharmaceutics. 2023. PMID: 36986751 Free PMC article.
-
Pharmacobezoar Formation From HPMC-AS-Containing Spray-Dried Formulations in Nonclinical Safety Studies in Rats.Toxicol Pathol. 2022 Dec;50(8):920-929. doi: 10.1177/01926233221145112. Epub 2022 Dec 21. Toxicol Pathol. 2022. PMID: 36541591
-
Correlationship of Drug-Polymer Miscibility, Molecular Relaxation and Phase Behavior of Dipyridamole Amorphous Solid Dispersions.J Pharm Sci. 2021 Apr;110(4):1470-1479. doi: 10.1016/j.xphs.2020.12.007. Epub 2020 Dec 15. J Pharm Sci. 2021. PMID: 33333143
-
Hydroxypropyl methylcellulose acetate succinate as an exceptional polymer for amorphous solid dispersion formulations: A review from bench to clinic.Eur J Pharm Biopharm. 2022 Aug;177:289-307. doi: 10.1016/j.ejpb.2022.07.010. Epub 2022 Jul 21. Eur J Pharm Biopharm. 2022. PMID: 35872180 Review.
-
Hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions: an overview.Mol Pharm. 2008 Nov-Dec;5(6):1003-19. doi: 10.1021/mp8000793. Mol Pharm. 2008. PMID: 19040386 Review.
Cited by
-
Evaluation of Pharmacobezoar Formation from Suspensions of Spray-Dried Amorphous Solid Dispersions: An MRI Study in Rats.Pharmaceutics. 2023 Mar 9;15(3):887. doi: 10.3390/pharmaceutics15030887. Pharmaceutics. 2023. PMID: 36986751 Free PMC article.
References
-
- ICH . ICH M3 (R2)—Non-Clinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorisation for Pharmaceuticals. ICH; Geneva, Switzerland: 2009.
LinkOut - more resources
Full Text Sources
Research Materials

