Carvacrol and HP-β-Cyclodextrin Complexes: Extensive Characterization and Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells
- PMID: 36559131
- PMCID: PMC9786748
- DOI: 10.3390/pharmaceutics14122638
Carvacrol and HP-β-Cyclodextrin Complexes: Extensive Characterization and Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells
Abstract
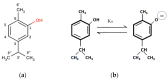
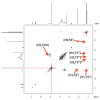
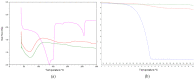
The aim of this study was to obtain solid carvacrol-cyclodextrin (CD) complexes for use in the pharmaceutical industry. To this end, the complexation of carvacrol at different pH values was studied in detail, to determine the type of CD and the reaction environment that supported the highest amount of encapsulated carvacrol. Evidence of the capability of hydroxypropyl-β-cyclodextrins (HP-β-CD) to form inclusion complexes with carvacrol (KC = 5042 ± 176 L mol-1) and more high complexation efficiency (2.824) was demonstrated for HP-β-CDs using two different energy sources, ultrasound (US) (KC = 8129 ± 194 L mol-1 24 h) and microwave irradiation (MWI) (KC = 6909 ± 161 L mol-1), followed by spraying the resulting solution in a spray dryer. To confirm complex formation, the complexes were characterized using various instrumental methods to corroborate the carvacrol incorporation into the hydrophobic cavity of HP-β-CD. The obtained carvacrol solid complexes were analyzed by 1H nuclear magnetic resonance (1H-NMR) and 2D nuclear magnetic resonance (ROSEY), differential scanning calorimetry (DSC), thermogravimetric analysis (TG) and Fourier transform infrared spectroscopy (FTIR) characterization. The structures of the resulting complexes were also characterized by molecular modeling. Furthermore, 1 mM HP-β-CD-carvacrol complex has been shown to reduce cell proliferation in HCT-116 colorectal cancer cells by 43%, much more than in a healthy lung fibroblast MRC-5 cell line (11%).
Keywords: HP-β-cyclodextrins; carvacrol; cell viability; chemical characterization; microwave irradiation; solid complexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Ni Z.-J., Wang X., Shen Y., Thakur K., Han J., Zhang J.-G., Hu F., Wei Z.-J. Recent Updates on the Chemistry, Bioactivities, Mode of Action, and Industrial Applications of Plant Essential Oils. Trends Food Sci. Technol. 2021;110:78–89. doi: 10.1016/j.tifs.2021.01.070. - DOI
-
- Evergetis E., Michaelakis A., Papachristos D.P., Badieritakis E., Kapsaski-Kanelli V.N., Haroutounian S.A. Seasonal Variation and Bioactivity of the Essential Oils of Two Juniperus Species against Aedes (Stegomyia) Albopictus (Skuse, 1894) Parasitol. Res. 2016;115:2175–2183. doi: 10.1007/s00436-016-4959-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

