Preliminary Assessment of Intramuscular Depot of Lipid-Based Decoquinate Formulation for Long-Term Chemoprophylaxis of Malaria
- PMID: 36559304
- PMCID: PMC9782194
- DOI: 10.3390/pharmaceutics14122813
Preliminary Assessment of Intramuscular Depot of Lipid-Based Decoquinate Formulation for Long-Term Chemoprophylaxis of Malaria
Abstract
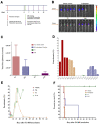
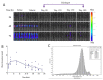
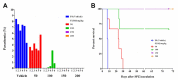
Sustained-release formulations of decoquinate were evaluated for the long-term prophylaxis of malaria. In the initial experiment, mice were protected from liver-stage Plasmodium infection by intramuscular administration of a lipids-based formulation at a dose of decoquinate 200 mg/kg. The mice that were inoculated with Plasmodium berghei sporozoites 34 days after the administration of a one-time drug dose were continuously monitored for 60 days and shown to be free of Plasmodium parasites. The optimized formulation for the sustained release of decoquinate was prepared by hot melt extrusion, constructed by lipids including cholesterol and mono or diglycerides, and had a drug load of 20 to 40% and particle size of 30 to 50 μm. Decoquinate of the lipids-based formulation was slowly released in vitro at a constant rate for the duration of two months, and was examined and continuously exposed at a therapeutic level in the blood for as long as 4 to 6 months. Further evaluation showed that the lipids-based formulation at doses of decoquinate 100 to 150 mg/kg could protect mice from Plasmodium infection for a period of 120 days. It is the first time that cholesterol has been used for a controlled drug delivery system of decoquinate. The results may provide useful information, not only for preparing a formulation of long-acting decoquinate but also in general for developing a controlled drug release system. The one-time administration of pharmaceutical agents in such a slow-release system may serve patients with no concerns about compliance.
Keywords: Plasmodium infection; cholesterol; decoquinate; sustained release.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Long-Term Prophylaxis and Pharmacokinetic Evaluation of Intramuscular Nano- and Microparticle Decoquinate in Mice Infected with P. berghei Sporozoites.Malar Res Treat. 2017;2017:7508291. doi: 10.1155/2017/7508291. Epub 2017 Apr 13. Malar Res Treat. 2017. PMID: 28491482 Free PMC article.
-
Preparation of Decoquinate Solid Dispersion by Hot-Melt Extrusion as an Oral Dosage Form Targeting Liver-Stage Plasmodium Infection.Antimicrob Agents Chemother. 2022 Jun 21;66(6):e0221821. doi: 10.1128/aac.02218-21. Epub 2022 Jun 6. Antimicrob Agents Chemother. 2022. PMID: 35658489 Free PMC article.
-
Nanoparticle formulations of decoquinate increase antimalarial efficacy against liver stage Plasmodium infections in mice.Nanomedicine. 2014 Jan;10(1):57-65. doi: 10.1016/j.nano.2013.07.010. Epub 2013 Jul 25. Nanomedicine. 2014. PMID: 23891618
-
Decoquinate liposomes: highly effective clearance of Plasmodium parasites causing severe malaria.Malar J. 2022 Jan 24;21(1):24. doi: 10.1186/s12936-022-04042-8. Malar J. 2022. PMID: 35073922 Free PMC article.
-
[Cost-effectiveness analysis of schizophrenic patient care settings: impact of an atypical antipsychotic under long-acting injection formulation].Encephale. 2005 Mar-Apr;31(2):235-46. doi: 10.1016/s0013-7006(05)82390-5. Encephale. 2005. PMID: 15959450 Review. French.
Cited by
-
Long-acting intramuscular injections of ELQ-331, an antimalarial agent.Eur J Pharm Sci. 2024 Jul 1;198:106795. doi: 10.1016/j.ejps.2024.106795. Epub 2024 May 8. Eur J Pharm Sci. 2024. PMID: 38729224 Free PMC article.
References
-
- WHO World Malaria Report 2020: 20 Years of Global Progress and Challenges. 2020. [(accessed on 23 September 2021)]. Available online: https://www.who.int/teams/globalmalaria-programme.
-
- Roth A., Maher S.P., Conway A.J., Ubalee R., Chaumeau V., Andolina C., Kaba S.A., Vantaux A., Vantaux M.A., Thomson-Luque R., et al. A comprehensive model for assessment of liver stage therapies targeting Plasmodium vivax and Plasmodium falciparum. Nat. Commun. 2018;9:1837. doi: 10.1038/s41467-018-04221-9. - DOI - PMC - PubMed
-
- March S., Ng S., Velmurugan S., Galstian A., Shan J., Logan D.J., Carpenter A.E., Thomas D., Sim B.K.L., Mota M.M., et al. A Microscale Human Liver Platform that Supports the Hepatic Stages of Plasmodium falciparum and vivax. Cell Host Microbe. 2013;14:104–115. doi: 10.1016/j.chom.2013.06.005. - DOI - PMC - PubMed
-
- DeVos E., Dunn N. StatPearls (Internet) StatPearls Publishing; Treasure Island, FL, USA: Jan, 2022. [(accessed on 4 July 2022)]. Malaria Prophylaxis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551639/ - PubMed
LinkOut - more resources
Full Text Sources

