MUPS Tableting-Comparison between Crospovidone and Microcrystalline Cellulose Core Pellets
- PMID: 36559308
- PMCID: PMC9785026
- DOI: 10.3390/pharmaceutics14122812
MUPS Tableting-Comparison between Crospovidone and Microcrystalline Cellulose Core Pellets
Abstract
Multi-unit pellet system (MUPS) tablets were fabricated by compacting drug-loaded pellets of either crospovidone or microcrystalline cellulose core. These pellets were produced by extrusion-spheronization and coated with ethylcellulose (EC) for a sustained drug release function. Coat damage due to the MUPS tableting process could undermine the sustained release function of the EC-coated pellets. Deformability of the pellet core is a factor that can impact the extent of pellet coat damage. Thus, this study was designed to evaluate the relative performance of drug-loaded pellets prepared with either microcrystalline cellulose (MCC) or crospovidone (XPVP) as a spheronization aid and were comparatively evaluated for their ability to withstand EC pellet coat damage when compacted. These pellets were tableted at various compaction pressures and pellet volume fractions. The extent of pellet coat damage was assessed by the change in drug release after compaction. The findings from this study demonstrated that pellets spheronized with XPVP had slightly less favorable physical properties and experienced comparatively more pellet coat damage than the pellets with MCC. However, MUPS tablets of reasonable quality could successfully be produced from pellets with XPVP, albeit their performance did not match that of vastly mechanically stronger pellets with MCC at higher compaction pressure.
Keywords: MUPS tablet; compaction energy; crospovidone; ethylcellulose; microcrystalline cellulose; pellet coat damage; pellet core; spheronization aid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



 ) and XPVP (
) and XPVP ( ) pellets contained in the MUPS tablet (n = 4). (a) Rearrangement energy, (b) plastic energy, (c) compression energy, and (d) ejection energy. Error bars represent the standard deviation. Trendlines (dotted) were fitted to discern trends. Control tablets (n = 5) acted as the baseline, and their compaction properties are indicated by a horizontal line (solid) with the bracketing as standard deviation lines (dashed).
) pellets contained in the MUPS tablet (n = 4). (a) Rearrangement energy, (b) plastic energy, (c) compression energy, and (d) ejection energy. Error bars represent the standard deviation. Trendlines (dotted) were fitted to discern trends. Control tablets (n = 5) acted as the baseline, and their compaction properties are indicated by a horizontal line (solid) with the bracketing as standard deviation lines (dashed).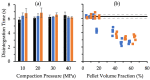

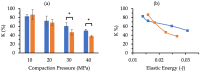

 ), II (
), II ( ), III (
), III ( ), IV (
), IV ( ), V (
), V ( ), VI (
), VI ( ), and VII (
), and VII ( ). Error bars represent the standard deviation (n = 4). Plots of (b) K and (c) IDR values against pellet volume fractions for MCC (
). Error bars represent the standard deviation (n = 4). Plots of (b) K and (c) IDR values against pellet volume fractions for MCC ( ) and XPVP (
) and XPVP ( ) pellets. Each symbol represents an individual sample. Approximated trendlines and critical pellet volume fractions are shown for the respective pellets using solid and dashed lines, respectively.
) pellets. Each symbol represents an individual sample. Approximated trendlines and critical pellet volume fractions are shown for the respective pellets using solid and dashed lines, respectively.References
-
- Mishra R.V., Paldewar S.G., Nandgude T.D. An Outline of Variables in Pelletization by Extrusion and Spheronization. Int. J. Appl. Pharm. 2020;12:39–44. doi: 10.22159/ijap.2020v12i4.37277. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

