Deferasirox Nanosuspension Loaded Dissolving Microneedles for Intradermal Delivery
- PMID: 36559310
- PMCID: PMC9784557
- DOI: 10.3390/pharmaceutics14122817
Deferasirox Nanosuspension Loaded Dissolving Microneedles for Intradermal Delivery
Abstract
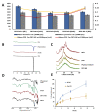
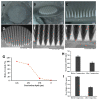
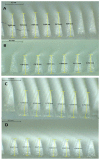
Microneedles are minimally invasive systems that can deliver drugs intradermally without pain and bleeding and can advantageously replace the hypodermal needles and oral routes of delivery. Deferasirox (DFS) is an iron chelator employed in several ailments where iron overload plays an important role in disease manifestation. In this study, DFS was formulated into a nanosuspension (NSs) through wet media milling employing PVA as a stabilizer and successfully loaded in polymeric dissolving microneedles (DMNs). The release studies for DFS-NS clearly showed a threefold increased dissolution rate compared to pure DFS. The mechanical characterization of DFS-NS-DMNs revealed that the system was sufficiently strong for efficacious skin penetration. Optical coherence tomography images confirmed an insertion of up to 378 µm into full-thickness porcine skin layers. The skin deposition studies showed 60% drug deposition from NS-DMN, which was much higher than from the DFS-NS transdermal patch (DFS-NS-TP) (without needles) or pure DFS-DMNs. Moreover, DFS-NS without DMNs did not deposit well inside the skin, indicating that DMNs played an important role in effectively delivering drugs inside the skin. Therefore, it is evident from the findings that loading DFS-NS into novel DMN devices can effectively deliver DFS transdermally.
Keywords: deferasirox; dissolving microneedles; intradermal delivery; nanocrystals; nanosuspension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Yadav P.R., Munni M.N., Campbell L., Mostofa G., Dobson L., Shittu M., Pattanayek S.K., Uddin M.J., Das D.B. Translation of Polymeric Microneedles for Treatment of Human Diseases: Recent Trends, Progress, and Challenges. Pharmaceutics. 2021;13:1132. doi: 10.3390/pharmaceutics13081132. - DOI - PMC - PubMed
-
- Volpe-Zanutto F., Ferreira L.T., Permana A.D., Kirkby M., Paredes A.J., Vora L.K., Bonfanti A.P., Charlie-Silva I., Raposo C., Figueiredo M.C., et al. Artemether and Lumefantrine Dissolving Microneedle Patches with Improved Pharmacokinetic Performance and Antimalarial Efficacy in Mice Infected with Plasmodium Yoelii. J. Control. Release. 2021;333:298–315. doi: 10.1016/j.jconrel.2021.03.036. - DOI - PubMed
-
- Vora L.K., Donnelly R.F., Larrañeta E., González-Vázquez P., Thakur R.R.S., Vavia P.R. Novel Bilayer Dissolving Microneedle Arrays with Concentrated PLGA Nano-Microparticles for Targeted Intradermal Delivery: Proof of Concept. J. Control. Release. 2017;265:93–101. doi: 10.1016/j.jconrel.2017.10.005. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

