Simulated Analysis of Influence of Changes in H+-ATPase Activity and Membrane CO2 Conductance on Parameters of Photosynthetic Assimilation in Leaves
- PMID: 36559546
- PMCID: PMC9783116
- DOI: 10.3390/plants11243435
Simulated Analysis of Influence of Changes in H+-ATPase Activity and Membrane CO2 Conductance on Parameters of Photosynthetic Assimilation in Leaves
Abstract
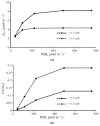
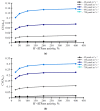
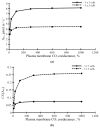
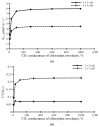
Photosynthesis is an important process in plants which influences their development and productivity. Many factors can control the efficiency of photosynthesis, including CO2 conductance of leaf mesophyll, which affects the CO2 availability for Rubisco. It is known that electrical stress signals can decrease this conductance, and the response is probably caused by inactivation of H+-ATPase in the plasma membrane. In the current work, we analyzed the influence of both CO2 conductance in the plasma membrane, and chloroplast envelopes and H+-ATPase activity on photosynthetic CO2 assimilation, using a two-dimensional mathematical model of photosynthesis in leaves. The model included a description of assimilation on the basis of the Farquhar-von Caemmerer-Berry model, ion transport through the plasma membrane, diffusion of CO2 in the apoplast, and transport of CO2 through the plasma membrane and chloroplast envelope. The model showed that the photosynthetic CO2 assimilation rate was mainly dependent on the plasma membrane and chloroplast envelope conductance; direct influence of the H+-ATPase activity (through changes in pH and CO2/HCO3- concentration ratio) on this rate was weak. In contrast, both changes in CO2 conductance of the plasma membrane and chloroplast envelopes and changes in the H+-ATPase activity influenced spatial heterogeneity of the CO2 assimilation on the leaf surface in the simulated two-dimensional system. These effects were also observed under simultaneous changes in the CO2 conductance of the plasma membrane and H+-ATPase activity. Qualitatively similar influence of changes in the CO2 conductance of the plasma membrane and chloroplast envelopes, and changes in the H+-ATPase activity on photosynthesis were shown for two different densities of stomata in the simulated leaf; however, lowering the density of stomata decreased the assimilation rate and increased the heterogeneity of assimilation. The results of the model analysis clarify the potential influence of H+-ATPase inactivation on photosynthesis, and can be the basis for development of new methods for remote sensing of the influence of electrical signals.
Keywords: H+-ATPase; chloroplast envelope CO2 conductance; electrical signals; photosynthetic CO2 assimilation; plasma membrane CO2 conductance; spatial heterogeneity; two-dimensional photosynthetic model.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, the collection, analyses, or interpretation of data, the writing of the manuscript or in the decision to publish the results.
Figures


References
-
- Von Caemmerer S., Farquhar G., Berry J. Biochemical model of C3 photosynthesis. In: Laisk A., Nedbal L., Govindjee, editors. Photosynthesis In Silico: Advances in Photosynthesis and Respiration. Volume 29. Springer; Dordrecht, The Netherlands: 2009. pp. 209–230.
Grants and funding
LinkOut - more resources
Full Text Sources

