TPC1-Type Channels in Physcomitrium patens: Interaction between EF-Hands and Ca2
- PMID: 36559639
- PMCID: PMC9783492
- DOI: 10.3390/plants11243527
TPC1-Type Channels in Physcomitrium patens: Interaction between EF-Hands and Ca2
Abstract
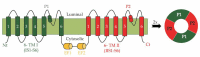
Two-pore channels (TPCs) are members of the superfamily of ligand-gated and voltage-sensitive ion channels in the membranes of intracellular organelles of eukaryotic cells. The evolution of ordinary plant TPC1 essentially followed a very conservative pattern, with no changes in the characteristic structural footprints of these channels, such as the cytosolic and luminal regions involved in Ca2+ sensing. In contrast, the genomes of mosses and liverworts encode also TPC1-like channels with larger variations at these sites (TPC1b channels). In the genome of the model plant Physcomitrium patens we identified nine non-redundant sequences belonging to the TPC1 channel family, two ordinary TPC1-type, and seven TPC1b-type channels. The latter show variations in critical amino acids in their EF-hands essential for Ca2+ sensing. To investigate the impact of these differences between TPC1 and TPC1b channels, we generated structural models of the EF-hands of PpTPC1 and PpTPC1b channels. These models were used in molecular dynamics simulations to determine the frequency with which calcium ions were present in a coordination site and also to estimate the average distance of the ions from the center of this site. Our analyses indicate that the EF-hand domains of PpTPC1b-type channels have a lower capacity to coordinate calcium ions compared with those of common TPC1-like channels.
Keywords: EF-domain; Physcomitrella; TPC1; coordination of Ca2+; vacuole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Differential contribution of EF-hands to the Ca²⁺-dependent activation in the plant two-pore channel TPC1.Plant J. 2011 Nov;68(3):424-32. doi: 10.1111/j.1365-313X.2011.04697.x. Epub 2011 Aug 18. Plant J. 2011. PMID: 21736651
-
Computational Analyses of the AtTPC1 (Arabidopsis Two-Pore Channel 1) Permeation Pathway.Int J Mol Sci. 2021 Sep 26;22(19):10345. doi: 10.3390/ijms221910345. Int J Mol Sci. 2021. PMID: 34638686 Free PMC article.
-
Gating of the two-pore cation channel AtTPC1 in the plant vacuole is based on a single voltage-sensing domain.Plant Biol (Stuttg). 2016 Sep;18(5):750-60. doi: 10.1111/plb.12478. Epub 2016 Jul 12. Plant Biol (Stuttg). 2016. PMID: 27270880
-
Two-Pore Channels Regulate Inter-Organellar Ca2+ Homeostasis in Immune Cells.Cells. 2022 Apr 26;11(9):1465. doi: 10.3390/cells11091465. Cells. 2022. PMID: 35563771 Free PMC article. Review.
-
Major vacuolar TPC1 channel in stress signaling: what matters, K+, Ca2+ conductance or an ion-flux independent mechanism?Stress Biol. 2022 Aug 11;2(1):31. doi: 10.1007/s44154-022-00055-0. Stress Biol. 2022. PMID: 37676554 Free PMC article. Review.
References
-
- Hedrich R., Neher E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature. 1987;329:833–836. doi: 10.1038/329833a0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

