Removal of Cationic Dyes by Iron Modified Silica/Polyurethane Composite: Kinetic, Isotherm and Thermodynamic Analyses, and Regeneration via Advanced Oxidation Process
- PMID: 36559783
- PMCID: PMC9786703
- DOI: 10.3390/polym14245416
Removal of Cationic Dyes by Iron Modified Silica/Polyurethane Composite: Kinetic, Isotherm and Thermodynamic Analyses, and Regeneration via Advanced Oxidation Process
Abstract
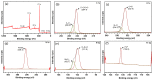
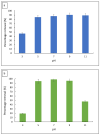
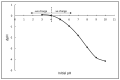
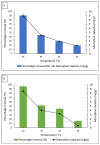
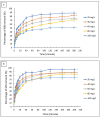
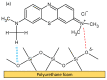
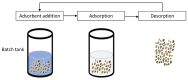
Emerging dye pollution from textile industrial effluents is becoming more challenging for researchers worldwide. The contamination of water by dye effluents affects the living organisms in an ecosystem. Methylene blue (MB) and malachite green (MG) are soluble dyes with a high colour intensity even at low concentration and are hazardous to living organisms. The adsorption method is used in most wastewater plants for the removal of organic pollutants as it is cost-effective, has a high adsorption capacity, and good mechanical stabilities. In this study, a composite adsorbent was prepared by impregnating iron modified silica (FMS) onto polyurethane (PU) foam to produce an iron modified silica/polyurethane (FMS/PU) composite. The composite adsorbent was utilised in batch adsorption of the cationic dyes MB and MG. The effect of adsorption parameters such as the adsorbent load, pH, initial dye concentration, and contact time were discussed. Adsorption kinetics and isotherm were implemented to understand the adsorption mechanism for both dyes. It was found that the adsorption of MB and MG followed the pseudo-second order model. The Langmuir model showed a better fit than the Freundlich model for the adsorption of MB and MG, indicating that the adsorption occurred via the monolayer adsorption system. The maximum adsorption capacity of the FMS/PU obtained for MB was 31.7 mg/g, while for MG, it was 34.3 mg/g. The thermodynamic study revealed that the adsorption of MB and MG were exothermic and spontaneous at room temperature. In addition, the regeneration of FMS/PU was conducted to investigate the composite efficiency in adsorbing dyes for several cycles. The results showed that the FMS/PU composite could be regenerated up to four times when the regeneration efficiency dropped drastically to less than 20.0%. The impregnation of FMS onto PU foam also minimised the adsorbent loss into the environment.
Keywords: adsorption; advanced oxidation process; iron modified silica; isotherm; kinetic; malachite green; methylene blue; polyurethane; regeneration; thermodynamic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Nie Q., Nie S. Evaluation Technologies for Food Quality. Elsevier; Amsterdam, The Netherlands: 2019. High-performance liquid chromatography for food quality evaluation; pp. 267–299. - DOI
-
- Tan B. Removal of dyes and industrial dye wastes by magnesium chloride. Water Res. 2000;34:597–601. doi: 10.1016/S0043-1354(99)00151-7. - DOI
-
- Anirudhan T.S., Ramachandran M. Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): Kinetic and competitive adsorption isotherm. Process Saf. Environ. Prot. 2015;95:215–225. doi: 10.1016/j.psep.2015.03.003. - DOI
-
- Pessier A.P. Current Therapy in Reptile Medicine and Surgery. Elsevier; Amsterdam, The Netherlands: 2014. Chytridiomycosis; pp. 255–270. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

