Polyester Nanocapsules for Intravenous Delivery of Artemether: Formulation Development, Antimalarial Efficacy, and Cardioprotective Effects In Vivo
- PMID: 36559869
- PMCID: PMC9786304
- DOI: 10.3390/polym14245503
Polyester Nanocapsules for Intravenous Delivery of Artemether: Formulation Development, Antimalarial Efficacy, and Cardioprotective Effects In Vivo
Abstract
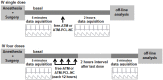
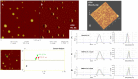
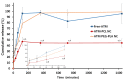
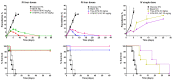
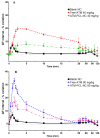
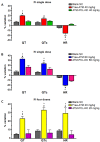
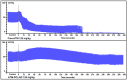
Artemether (ATM) is an effective antimalarial drug that also has a short half-life in the blood. Furthermore, ATM is also cardiotoxic and is associated with pro-arrhythmogenic risks. We aimed to develop a delivery system enabling the prolonged release of ATM into the blood coupled with reduced cardiotoxicity. To achieve this, we prepared polymeric nanocapsules (NCs) from different biodegradable polyesters, namely poly(D,L-lactide) (PLA), poly-ε-caprolactone (PCL), and surface-modified NCs, using a monomethoxi-polyethylene glycol-block-poly(D,L-lactide) (PEG5kDa-PLA45kDa) polymer. Using this approach, we were able to encapsulate high yields of ATM (>85%, 0−4 mg/mL) within the oily core of the NCs. The PCL-NCs exhibited the highest percentage of ATM loading as well as a slow release rate. Atomic force microscopy showed nanometric and spherical particles with a narrow size dispersion. We used the PCL NCs loaded with ATM for biological evaluation following IV administration. As with free-ATM, the ATM-PCL-NCs formulation exhibited potent antimalarial efficacy using either the “Four-day test” protocol (ATM total at the end of the 4 daily doses: 40 and 80 mg/kg) in Swiss mice infected with P. berghei or a single low dose (20 mg/kg) of ATM in mice with higher parasitemia (15%). In healthy rats, IV administration of single doses of free-ATM (40 or 80 mg/kg) prolonged cardiac QT and QTc intervals and induced both bradycardia and hypotension. Repeated IV administration of free-ATM (four IV doses at 20 mg/kg every 12 h for 48 h) also prolonged the QT and QTc intervals but, paradoxically, induced tachycardia and hypertension. Remarkably, the incorporation of ATM in ATM-PCL-NCs reduced all adverse effects. In conclusion, the encapsulation of ATM in biodegradable polyester NCs reduces its cardiovascular toxicity without affecting its antimalarial efficacy.
Keywords: QT interval; artemether; cardiotoxicity; drug delivery; malaria; nanocapsules; polylactide; self-assembled polymers.
Conflict of interest statement
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Figures


References
-
- World Health Organization World Malaria Report. Geneva, Switzerland, 2021. License: CC BY-NC-SA 3.0 IGO 2021. [(accessed on 22 November 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria.
-
- World Health Organization . World Malaria Report 2020: 20 Years of Global Progress and Challenges. WHO; Geneva, Switzerland: 2020.
-
- Volpe-Zanutto F., Ferreira L.T., Permana A.D., Kirkby M., Paredes A.J., Vora L.K., Bonfanti A.P., Charlie-Silva I., Raposo C., Figueiredo M.C., et al. Artemether and lumefantrine dissolving microneedle patches with improved pharmacokinetic performance and antimalarial efficacy in mice infected with Plasmodium yoelii. J. Control Release. 2021;333:298–315. doi: 10.1016/j.jconrel.2021.03.036. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

