Potentiometric Hydrogen Sensor with 3D-Printed BaCe0.6Zr0.3Y0.1O3-α Electrolyte for High-Temperature Applications
- PMID: 36560077
- PMCID: PMC9785787
- DOI: 10.3390/s22249707
Potentiometric Hydrogen Sensor with 3D-Printed BaCe0.6Zr0.3Y0.1O3-α Electrolyte for High-Temperature Applications
Abstract
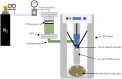
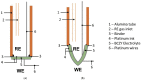
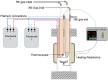
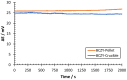
Hydrogen is expected to play an important role in the near future in the transition to a net-zero economy. Therefore, the development of new in situ and real-time analytical tools able to quantify hydrogen at high temperatures is required for future applications. Potentiometric sensors based on perovskite-structured solid-state electrolytes can be a good option for H2 monitoring. Nevertheless, the geometry of the sensor should be designed according to the specific necessities of each technological field. Conventional shaping processes need several iterations of green shaping and machining to achieve a good result. In contrast, 3D printing methods stand out from conventional ones since they simplify the creation of prototypes, reducing the cost and the number of iterations needed for the obtainment of the final design. In the present work, BaCe0.6Zr0.3Y0.1O3-α (BCZY) was used as a proton-conducting electrolyte for potentiometric sensors construction. Two different shapes were tested for the sensors' electrolyte: pellets (BCZY-Pellet) and crucibles (BCZY-Crucible). Ceramics were shaped using extrusion-based 3D printing. Finally, parameters, such as sensitivity, response time, recovery time and the limit of detection and accuracy, were evaluated for both types of sensors (BCZY-Pellet and BCZY-Crucible) at 500 °C.
Keywords: BCZY; BaCe0.6Zr0.3Y0.1O3-α; ceramic 3D printing; perovskite; potentiometric sensor; proton-conducting materials.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- Singh S., Jain S., PS V., Tiwari A.K., Nouni M.R., Pandey J.K., Goel S. Hydrogen: A Sustainable Fuel for Future of the Transport Sector. Renew. Sustain. Energy Rev. 2015;51:623–633. doi: 10.1016/j.rser.2015.06.040. - DOI
-
- Reddy S.N., Nanda S., Vo D.-V.N., Nguyen T.D., Nguyen V.-H., Abdullah B., Nguyen-Tri P. New Dimensions in Production and Utilization of Hydrogen. Elsevier; Amsterdam, The Netherlands: 2020. Hydrogen: Fuel of the near Future; pp. 1–20.
-
- Rievaj V., Gaňa J., Synák F. Is Hydrogen the Fuel of the Future? Transp. Res. Procedia. 2019;40:469–474. doi: 10.1016/j.trpro.2019.07.068. - DOI
-
- Møller K.T., Jensen T.R., Akiba E., Li H. Hydrogen—A Sustainable Energy Carrier. Prog. Nat. Sci.: Mater. Int. 2017;27:34–40. doi: 10.1016/j.pnsc.2016.12.014. - DOI
-
- Zhang X., Chan S.H., Li G., Ho H.K., Li J., Feng Z. A Review of Integration Strategies for Solid Oxide Fuel Cells. J Power Sources. 2010;195:685–702. doi: 10.1016/j.jpowsour.2009.07.045. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

