Pilot Findings on SARS-CoV-2 Vaccine-Induced Pituitary Diseases: A Mini Review from Diagnosis to Pathophysiology
- PMID: 36560413
- PMCID: PMC9786744
- DOI: 10.3390/vaccines10122004
Pilot Findings on SARS-CoV-2 Vaccine-Induced Pituitary Diseases: A Mini Review from Diagnosis to Pathophysiology
Abstract
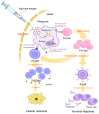
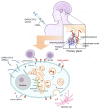
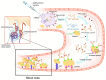
Since the emergence of the COVID-19 pandemic at the end of 2019, a massive vaccination campaign has been undertaken rapidly and worldwide. Like other vaccines, the COVID-19 vaccine is not devoid of side effects. Typically, the adverse side effects of vaccination include transient headache, fever, and myalgia. Endocrine organs are also affected by adverse effects. The major SARS-CoV-2 vaccine-associated endocrinopathies reported since the beginning of the vaccination campaign are thyroid and pancreas disorders. SARS-CoV-2 vaccine-induced pituitary diseases have become more frequently described in the literature. We searched PubMed/MEDLINE for commentaries, case reports, and case series articles reporting pituitary disorders following SARS-CoV-2 vaccination. The search was reiterated until September 2022, in which eight case reports were found. In all the cases, there were no personal or familial history of pituitary disease described. All the patients described had no previous SARS-CoV-2 infection prior to the vaccination episode. Regarding the type of vaccines administered, 50% of the patients received (BNT162b2; Pfizer-BioNTech) and 50% received (ChAdOx1 nCov-19; AstraZeneca). In five cases, the pituitary disorder developed after the first dose of the corresponding vaccine. Regarding the types of pituitary disorder, five were hypophysitis (variable clinical aspects ranging from pituitary lesion to pituitary stalk thickness) and three were pituitary apoplexy. The time period between vaccination and pituitary disorder ranged from one to seven days. Depending on each case's follow-up time, a complete remission was obtained in all the apoplexy cases but in only three patients with hypophysitis (persistence of the central diabetes insipidus). Both quantity and quality of the published data about pituitary inconveniences after COVID-19 vaccination are limited. Pituitary disorders, unlike thyroid disorders, occur very quickly after COVID-19 vaccination (less than seven days for pituitary disorders versus two months for thyroid disease). This is partially explained by the ease of reaching the pituitary, which is a small gland. Therefore, this gland is rapidly overspread, which explains the speed of onset of pituitary symptoms (especially ADH deficiency which is a rapid onset deficit with evocative symptoms). Accordingly, these pilot findings offer clinicians a future direction to be vigilant for possible pituitary adverse effects of vaccination. This will allow them to accurately orient patients for medical assistance when they present with remarkable symptoms, such as asthenia, polyuro-polydipsia, or severe headache, following a COVID-19 vaccination.
Keywords: ASIA syndrome; COVID-19; SARS-CoV-2; VITT; apoplexy; hypophysitis; pituitary; vaccine; vaccine-induced thrombotic thrombocytopenia.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

