Resistance towards ChadOx1 nCoV-19 in an 83 Years Old Woman Experiencing Vaccine Induced Thrombosis with Thrombocytopenia Syndrome
- PMID: 36560466
- PMCID: PMC9781243
- DOI: 10.3390/vaccines10122056
Resistance towards ChadOx1 nCoV-19 in an 83 Years Old Woman Experiencing Vaccine Induced Thrombosis with Thrombocytopenia Syndrome
Abstract
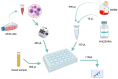
Background: in this report, we describe the case of an 83-year-old woman vaccinated with ChadOx1 nCoV-19 who developed a so-called vaccine-induced thrombosis with thrombocytopenia syndrome and who did not develop any antibodies against the spike protein of SARS-CoV-2 at 30 days following the administration of her first dose of ChadOx1 nCoV-19. Experimental section: two serum samples from the patient and 5 serum samples from 5 control individuals having received the two-dose regimen vaccination with ChadOx1 nCoV-19 were evaluated. In order to investigate the lack of response to the vaccination, a cell model was developed. This model permits to evaluate the interaction between responsive cells (A549) possessing the Coxsackievirus and Adenovirus Receptor (CAR), a defined concentration of ChadOx1 nCoV-19 and serial dilution of the patient or the control serum. The aim was to assess the impact of these sera on the production of the spike (S) protein induced by the transfection of the genetic material of ChadOx1 nCoV-19 into the A549 cells. The S protein is measured in the supernatant using an ELISA technique.
Results: interestingly, the serum from the patient who developed the vaccine-induced thrombosis with thrombocytopenia syndrome impaired the production of S protein by the A549 cells transfected with ChadOx1 nCoV-19. This was not observed with the controls who did not interfere with the transfection of ChadOx1 nCoV-19 into A549 cells since the S protein is retrieved in the supernatant fraction.
Conclusion: based on the data coming from the clinical and the cell model information, we found a possible explanation on the absence of antibody response in our patient. She has, or has developed, characteristics that prevent the production of the S protein in contrast to control subjects. We were not able to investigate the entire mechanism behind this resistance which deserve further investigations. A link between this resistance and the development of the thrombosis with thrombocytopenia syndrome following vaccination with ChadOx1 nCoV-19 cannot be excluded.
Keywords: SARS-CoV-2; adenovirus; diagnostic; resistance; vaccine.
Conflict of interest statement
Among the authors, J.D. is CEO and founder of QUALIblood s.a., a contract research organization manufacturing the DP-Filter, is co-inventor of the DP-Filter (patent application number: PCT/ET2019/052903) and reports personal fees from Daiichi-Sankyo, Mithra Pharmaceuticals, Stago, Roche and Roche Diagnostics outside the submitted work. The other authors have no conflict of interest to disclose.
Figures

References
-
- Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., Li J.-X., Yang B.-F., Wang L., Wang W.-J., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

