Efficacy Studies against PCV-2 of a New Trivalent Vaccine including PCV-2a and PCV-2b Genotypes and Mycoplasma hyopneumoniae When Administered at 3 Weeks of Age
- PMID: 36560518
- PMCID: PMC9784864
- DOI: 10.3390/vaccines10122108
Efficacy Studies against PCV-2 of a New Trivalent Vaccine including PCV-2a and PCV-2b Genotypes and Mycoplasma hyopneumoniae When Administered at 3 Weeks of Age
Abstract
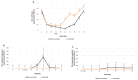
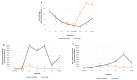
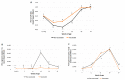
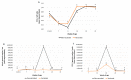
This study aimed to evaluate the efficacy of a new trivalent vaccine containing inactivated Porcine Circovirus 1-2a and 1-2b chimeras and a Mycoplasma hyopneumoniae bacterin administered to pigs around 3 weeks of age. This trivalent vaccine has already been proved as efficacious in a split-dose regimen but has not been tested in a single-dose scenario. For this purpose, a total of four studies including two pre-clinical and two clinical studies were performed. Globally, a significant reduction in PCV-2 viraemia and faecal excretion was detected in vaccinated pigs compared to non-vaccinated animals, as well as lower histopathological lymphoid lesion plus PCV-2 immunohistochemistry scorings, and incidence of PCV-2-subclinical infection. Moreover, in field trial B, a significant increase in body weight and in average daily weight gain were detected in vaccinated animals compared to the non-vaccinated ones. Circulation of PCV-2b in field trial A and PCV-2a plus PCV-2d in field trial B was confirmed by virus sequencing. Hence, the efficacy of this new trivalent vaccine against a natural PCV-2a, PCV-2b or PCV-2d challenge was demonstrated in terms of reduction of histopathological lymphoid lesions and PCV-2 detection in tissues, serum and faeces, as well as improvement of production parameters.
Keywords: efficacy; porcine circovirus 2; porcine respiratory disease complex; postweaning multisystemic wasting disease; single dose; trivalent vaccine.
Conflict of interest statement
J.C.M.G., M.F., S.B., G.S., L.T., M.B. (Meggan Bandrick), J.S. (Jennifer Smith) and M.B. (Mónica Balasch) are Zoetis employees. The rest of the authors declare no conflict of interest.
Figures

References
-
- Tassis P.D., Tsakmakidis I., Papatsiros V.G., Koulialis D., Nell T., Brellou G., Tzika E.D. A randomized controlled study on the efficacy of a novel combination vaccine against enzootic pneumonia (Mycoplasma hyopneuoniae) and porcine Circovirus type 2 (PCV2) in the presence of strong maternally derived PCV2 immunity in pigs. BMC Vet. Res. 2017;13:91. doi: 10.1186/s12917-017-1014-7. - DOI - PMC - PubMed
-
- Young M.G., Cunningham G.L., Sanford S.E. Circovirus vaccination in pigs with subclinical porcine circovirus type 2 infection complicated by ileitis. J. Swine Health Prod. 2011;19:175–180.
-
- Harding J.C.S., Clark E.G. Recognizing and diagnosing postweaning multisystemic wasting syndrome (PMWS) Swine Health Prod. 1997;5:201–203.
-
- Rosell C., Segalés J., Plana-Durán J., Balasch M., Rodríguez-Arrioja G.M., Kennedy S., Allan G.M., McNeilly F., Latimer K.S., Domingo M. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J. Comp. Pathol. 1999;120:59–78. doi: 10.1053/jcpa.1998.0258. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

