Immunostimulating Effect of Inactivated Parapoxvirus Ovis on the Serological Response to Equine Influenza Booster Vaccination
- PMID: 36560549
- PMCID: PMC9782193
- DOI: 10.3390/vaccines10122139
Immunostimulating Effect of Inactivated Parapoxvirus Ovis on the Serological Response to Equine Influenza Booster Vaccination
Abstract
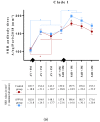
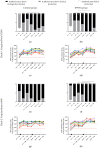
Equine influenza virus (EIV) is responsible for recurring outbreaks that are detrimental to the equine industry. Vaccination is key for prevention, but the effectiveness and duration of protection provided by existing vaccines is often insufficient. In order to improve vaccine efficacy, we evaluated the benefit of immune stimulation with inactivated Parapoxvirus ovis (iPPVO) on the antibody response induced by a vaccine boost against EIV. A whole inactivated ISCOMatrix-adjuvanted equine influenza vaccine was administered alone (n = 10) or combined with iPPVO injections at D0, D2 and D4 post vaccination (n = 10) to adult horses that required a vaccine boost 6 months after the last immunization, as now recommended by the WOAH. Antibody levels were measured with the single radial haemolysis (SRH) assay at 1, 3 and 6 months post-vaccination. Results revealed that horses that received iPPVO had higher antibody levels than the control group injected with the EI vaccine alone. Although the vaccine used contains only a clade 1 and European lineage strain, the increase in protective antibodies was also observed against a clade 2 strain. Thus, immune stimulation with iPPVO, a substance already marketed as an immunostimulant, could be used to improve vaccination protocols in horses and potentially other species.
Keywords: equine influenza viruses; horse; immunomodulator; inactivated Parapoxvirus ovis; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cullinane A. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE—World Organisation for Animal Health; Paris, France: 2022. [(accessed on 8 December 2022)]. Equine influenza (infection with equine influenza virus) Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.06.07_EQ....
-
- Fougerolle S., Fortier C., Legrand L., Jourdan M., Pitel C.M., Pronost S., Paillot R. Success and Limitation of Equine Influenza Vaccination: The First Incursion in a Decade of a Florida Clade 1 Equine Influenza Virus That Shakes Protection Despite High Vaccine Coverage. Vaccines. 2019;7:174. doi: 10.3390/vaccines7040174. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

