Comparison of a Prototype SARS-CoV-2 Lateral Flow IMMUNOASSAY with the BinaxNOWTM COVID-19 Antigen CARD
- PMID: 36560613
- PMCID: PMC9786212
- DOI: 10.3390/v14122609
Comparison of a Prototype SARS-CoV-2 Lateral Flow IMMUNOASSAY with the BinaxNOWTM COVID-19 Antigen CARD
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus responsible for the COVID-19 pandemic. From the onset of the pandemic, rapid antigen tests have quickly proved themselves to be an accurate and accessible diagnostic platform. The initial (and still most commonly used antigen tests) for COVID-19 diagnosis were constructed using monoclonal antibodies (mAbs) specific to severe acute respiratory syndrome coronavirus (SARS-CoV) nucleocapsid protein (NP). These mAbs are able to bind SARS-CoV-2 NP due to high homology between the two viruses. However, since first being identified in 2019, SARS-CoV-2 has continuously mutated, and a multitude of variants have appeared. These mutations have an elevated risk of leading to possible diagnostic escape when using tests produced with SARS-CoV-derived mAbs. Here, we established a library of 18 mAbs specific to SARS-CoV-2 NP and used two of these mAbs (1CV7 and 1CV14) to generate a prototype antigen-detection lateral flow immunoassay (LFI). A side-by-side analysis of the 1CV7/1CV14 LFI and the commercially available BinaxNOWTM COVID-19 Antigen CARD was performed. Results indicated the 1CV7/1CV14 LFI outperformed the BinaxNOWTM test in the detection of BA.2, BA.2.12.1, and BA.5 Omicron sub-variants when testing remnant RT-PCR positive patient nasopharyngeal swabs diluted in viral transport media.
Keywords: COVID-19; SARS-CoV-2; antigen assays; lateral flow immunoassay (LFI); monoclonal antibodies (mAb).
Conflict of interest statement
Amanda R. Burnham-Marusich and David P. AuCoin are shareholders of DxDiscovery, Inc. The remaining authors have no conflict of interest to declare.
Figures

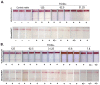


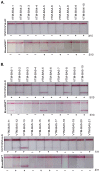
Similar articles
-
Monoclonal Antibodies against Nucleocapsid Protein of SARS-CoV-2 Variants for Detection of COVID-19.Int J Mol Sci. 2021 Nov 17;22(22):12412. doi: 10.3390/ijms222212412. Int J Mol Sci. 2021. PMID: 34830291 Free PMC article.
-
Methods to evaluate the impact of SARS-CoV-2 nucleocapsid mutations on antigen detection by rapid diagnostic tests.Biotechniques. 2022 Sep;73(3):136-141. doi: 10.2144/btn-2022-0020. Epub 2022 Aug 25. Biotechniques. 2022. PMID: 36004516 Free PMC article.
-
The development of a highly sensitive and quantitative SARS-CoV-2 rapid antigen test applying newly developed monoclonal antibodies to an automated chemiluminescent flow-through membrane immunoassay device.BMC Immunol. 2023 Sep 26;24(1):34. doi: 10.1186/s12865-023-00567-y. BMC Immunol. 2023. PMID: 37752417 Free PMC article.
-
Current status of the lateral flow immunoassay for the detection of SARS-CoV-2 in nasopharyngeal swabs.Biochem Med (Zagreb). 2021 Jun 15;31(2):020601. doi: 10.11613/BM.2021.020601. Biochem Med (Zagreb). 2021. PMID: 34140830 Free PMC article. Review.
-
Effectiveness of Abbott BinaxNOW Rapid Antigen Test for Detection of SARS-CoV-2 Infections in Outbreak among Horse Racetrack Workers, California, USA.Emerg Infect Dis. 2021 Nov;27(11):2761-2767. doi: 10.3201/eid2711.211449. Epub 2021 Sep 1. Emerg Infect Dis. 2021. PMID: 34469287 Free PMC article. Review.
References
-
- WHO Coronavirus (COVID-19) Dashboard WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. [(accessed on 8 June 2022)]; Available online: https://covid19.who.int/
-
- U.S. Food and Drug Administration (FDA) COVID-19 Vaccines. [(accessed on 6 April 2022)]; Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise....
-
- U.S. Food and Drug Administration . FDA Combating COVID-19 With Therapeutics. US Food and Drug Administration; Silver Spring, MD, USA: 2020.
-
- U.S. Food and Drug Administration (FDA) In Vitro Diagnostics EUAs. [(accessed on 6 April 2022)]; Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

