Insights from the Infection Cycle of VSV-ΔG-Spike Virus
- PMID: 36560832
- PMCID: PMC9788095
- DOI: 10.3390/v14122828
Insights from the Infection Cycle of VSV-ΔG-Spike Virus
Abstract
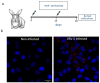
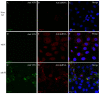
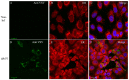
Fundamental key processes in viral infection cycles generally occur in distinct cellular sites where both viral and host factors accumulate and interact. These sites are usually termed viral replication organelles, or viral factories (VF). The generation of VF is accompanied by the synthesis of viral proteins and genomes and involves the reorganization of cellular structure. Recently, rVSV-ΔG-spike (VSV-S), a recombinant VSV expressing the SARS-CoV-2 spike protein, was developed as a vaccine candidate against SARS-CoV-2. By combining transmission electron microscopy (TEM) tomography studies and immuno-labeling techniques, we investigated the infection cycle of VSV-S in Vero E6 cells. RT-real-time-PCR results show that viral RNA synthesis occurs 3-4 h post infection (PI), and accumulates as the infection proceeds. By 10-24 h PI, TEM electron tomography results show that VSV-S generates VF in multi-lamellar bodies located in the cytoplasm. The VF consists of virus particles with various morphologies. We demonstrate that VSV-S infection is associated with accumulation of cytoplasmatic viral proteins co-localized with dsRNA (marker for RNA replication) but not with ER membranes. Newly formed virus particles released from the multi-lamellar bodies containing VF, concentrate in a vacuole membrane, and the infection ends with the budding of particles after the fusion of the vacuole membrane with the plasma membrane. In summary, the current study describes detailed 3D imaging of key processes during the VSV-S infection cycle.
Keywords: RNA virus; electron tomography; spike protein; transmission electron microscopy; vesicular stomatitis virus; viral factories.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

