Multicenter dose-escalation Phase I trial of mitomycin C pressurized intraperitoneal aerosolized chemotherapy in combination with systemic chemotherapy for appendiceal and colorectal peritoneal metastases: rationale and design
- PMID: 36560966
- PMCID: PMC9742457
- DOI: 10.1515/pp-2022-0116
Multicenter dose-escalation Phase I trial of mitomycin C pressurized intraperitoneal aerosolized chemotherapy in combination with systemic chemotherapy for appendiceal and colorectal peritoneal metastases: rationale and design
Abstract
Objectives: Peritoneal metastasis (PM) from appendiceal cancer or colorectal cancer (CRC) has significant morbidity and limited survival. Pressurized intraperitoneal aerosolized chemotherapy (PIPAC) is a minimally invasive approach to treat PM. We aim to conduct a dose-escalation trial of mitomycin C (MMC)-PIPAC combined with systemic chemotherapy (FOLFIRI) in patients with PM from appendiceal cancer or CRC.
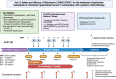
Methods: This is a multicenter Phase I study of MMC-PIPAC (NCT04329494). Inclusion criteria include treatment with at least 4 months of first- or second-line systemic chemotherapy with ineligibility for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC). Exclusion criteria are: progression on chemotherapy; extraperitoneal metastases; systemic chemotherapy intolerance; bowel obstruction; or poor performance status (ECOG>2). Escalating MMC-PIPAC doses (7-25 mg/m2) will be administered in combination with standard dose systemic FOLFIRI. Safety evaluation will be performed on 15 patients (dose escalation) and six expansion patients: 21 evaluable patients total.
Results: The primary endpoints are recommended MMC dose and safety of MMC-PIPAC with FOLFIRI. Secondary endpoints are assessment of response (by peritoneal regression grade score; Response Evaluation Criteria in Solid Tumors [RECIST 1.1], and peritoneal carcinomatosis index), progression free survival, overall survival, technical failure rate, surgical complications, conversion to curative-intent CRS-HIPEC, patient-reported outcomes, and functional status. Longitudinal blood and tissue specimens will be collected for translational correlatives including pharmacokinetics, circulating biomarkers, immune profiling, and single-cell transcriptomics.
Conclusions: This Phase I trial will establish the recommended dose of MMC-PIPAC in combination with FOLFIRI. Additionally, we expect to detect an early efficacy signal for further development of this therapeutic combination.
Keywords: Phase I study; appendiceal cancer; colorectal cancer (CRC); mitomycin C (MMC); peritoneal metastasis (PM); pressurized intraperitoneal aerosolized chemotherapy (PIPAC).
© 2022 the author(s), published by De Gruyter, Berlin/Boston.
Conflict of interest statement
Competing interests: Authors state no conflict of interest.
Figures
References
-
- Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. 2016;17:1709–19. doi: 10.1016/s1470-2045(16)30500-9. - DOI - PubMed
-
- Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, Phase 3 trial. Lancet Oncol. 2021;22:256–66. doi: 10.1016/s1470-2045(20)30599-4. - DOI - PubMed
-
- Rovers KP, Bakkers C, Nienhuijs SW, Burger JWA, Creemers G-JM, Thijs AMJ, et al. Perioperative systemic therapy vs cytoreductive surgery and hyperthermic intraperitoneal chemotherapy alone for resectable colorectal peritoneal metastases: a Phase 2 randomized clinical trial. JAMA Surg. 2021;156:710–20. - PMC - PubMed
-
- Girshally R, Demtröder C, Albayrak N, Zieren J, Tempfer C, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2016;14:253. doi: 10.1186/s12957-016-1008-0. - DOI - PMC - PubMed
-
- Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, Tempfer C, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21:553–9. doi: 10.1245/s10434-013-3213-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
