Discovery of small molecule mechanistic target of rapamycin inhibitors as anti-aging and anti-cancer therapeutics
- PMID: 36561137
- PMCID: PMC9767416
- DOI: 10.3389/fnagi.2022.1048260
Discovery of small molecule mechanistic target of rapamycin inhibitors as anti-aging and anti-cancer therapeutics
Abstract
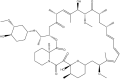
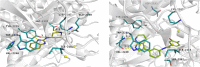
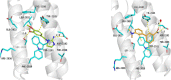
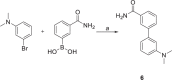
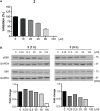
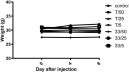
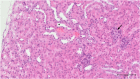
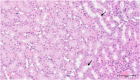
To date, the most studied drug in anti-aging research is the mTOR inhibitor - rapamycin. Despite its almost perfect anti-aging profile, rapamycin exerts one significant limitation - inappropriate physicochemical properties. Therefore, we have decided to utilize virtual high-throughput screening and fragment-based design in search of novel mTOR inhibiting scaffolds with suitable physicochemical parameters. Seven lead compounds were selected from the list of obtained hits that were commercially available (4, 5, and 7) or their synthesis was feasible (1, 2, 3, and 6) and evaluated in vitro and subsequently in vivo. Of all these substances, only compound 3 demonstrated a significant cytotoxic, senolytic, and senomorphic effect on normal and cancerous cells. Further, it has been confirmed that compound 3 is a direct mTORC1 inhibitor. Last but not least, compound 3 was found to exhibit anti-SASP activity concurrently being relatively safe within the test of in vivo tolerability. All these outstanding results highlight compound 3 as a scaffold worthy of further investigation.
Keywords: SASP phenotype; aging; anti-aging therapy; cancer; mTOR.
Copyright © 2022 Chrienova, Rysanek, Oleksak, Stary, Bajda, Reinis, Mikyskova, Novotny, Andrys, Skarka, Vasicova, Novak, Valis, Kuca, Hodny and Nepovimova.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
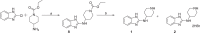






References
LinkOut - more resources
Full Text Sources
Miscellaneous

