Establishment of an efficient regeneration and Agrobacterium transformation system in mature embryos of calla lily (Zantedeschia spp.)
- PMID: 36561313
- PMCID: PMC9763309
- DOI: 10.3389/fgene.2022.1085694
Establishment of an efficient regeneration and Agrobacterium transformation system in mature embryos of calla lily (Zantedeschia spp.)
Abstract
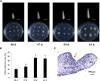
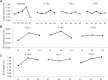
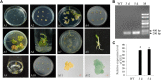
Calla lily (Zantedeschia spp.) have great aesthetic value due to their spathe-like appearance and richness of coloration. However, embryonic callus regeneration is absent from its current regeneration mechanism. As a result, constructing an adequate and stable genetic transformation system is hampered, severely hindering breeding efforts. In this research, the callus induction effectiveness of calla lily seed embryos of various maturities was evaluated. The findings indicated that mature seed embryos were more suitable for in vitro regeneration. Using orthogonal design experiments, the primary elements influencing in vitro regeneration, such as plant growth regulators, genotypes, and nanoscale materials, which was emergent uses for in vitro regeneration, were investigated. The findings indicated that MS supplemented with 6-BA 2 mg/L and NAA 0.1 mg/L was the optimal medium for callus induction (CIM); the germination medium (GM) was MS supplemented with 6-BA 2 mg/L NAA 0.2 mg/L and 1 mg/L CNTs, and the rooting medium (RM) was MS supplemented with 6-BA 2 mg/L NAA 0.7 mg/L and 2 mg/L CNTs. This allowed us to verify, in principle, that the Agrobacterium tumefaciens-mediated genetic transformation system operates under optimal circumstances using the GUS reporter gene. Here, we developed a seed embryo-based genetic transformation regeneration system, which set the stage for future attempts to create new calla lily varieties.
Keywords: CNTs; calla lily; genetic transformation; regeneration system; seed embryo.
Copyright © 2022 Sun, Wang, Yang, Wang, Wang, Wang, Liu, Wang, Zhang and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Abumhadi N., Kamenarova K., Todorovska E., Dimov G., Trifonova A., Gecheff K., et al. (2005). Callus induction and plant regeneration from barley mature embryos (Hordem vulgare L). Biotechnol. Biotechnol. Equip. 19 (3), 32–38. 10.1080/13102818.2005.10817224 - DOI
-
- Aggarwal D., Kumar A., Reddy M. S. (2011). Agrobacterium tumefaciens mediated genetic transformation of selected elite clone(s) of Eucalyptus tereticornis. Acta Physiol. Plant. 33, 1603–1611. 10.1007/s11738-010-0695-3 - DOI
-
- Beruto M., Lanteri L., Portogallo C. (2004). Micropropagation of tree peony (Paeonia suffruticosa). Plant Cell Tissue Organ Cult. 79, 249–255. 10.1007/s11240-004-0666-8 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

