Tear-derived exosomal biomarkers of Graves' ophthalmopathy
- PMID: 36561758
- PMCID: PMC9763563
- DOI: 10.3389/fimmu.2022.1088606
Tear-derived exosomal biomarkers of Graves' ophthalmopathy
Abstract
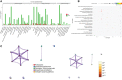
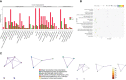
Graves' ophthalmopathy (GO), the most frequent extrathyroidal manifestation of Graves' disease (GD), can lead to a significant decline in the quality of life in patients. Exosomes, which contain proteins, lipids and DNA, play important roles in the pathological processes of various diseases. However, their roles in Graves' ophthalmopathy are still unclear. We aimed to isolate exosomes and analyze the different exosomal proteins. Tear fluids were collected from twenty-four GO patients, twenty-four GD patients and sixteen control subjects. The numbers of tear exosomes were assayed using nanoparticle tracking analysis. A Luminex 200 kit and ELISA kit were used to confirm the different cytokine concentrations in serum. Extraocular muscle from GO patients and controls was extracted, and western blotting was used to assay the levels of Caspase-3 and complement C4A. Our study demonstrated that the number of tear exosomes differ from GD patients and control. The expression levels of cytokines, including IL-1 and IL-18, were significantly increased in the tear exosomes and serum from GO patients compared with GD patients and controls. The levels of the exosomal proteins Caspase-3, complement C4A and APOA-IV were significantly increased in GO patients compared to GD patients and controls. Orbital fibroblasts from GO patients showed significantly higher levels of Caspase-3 and complement C4A than those from controls. The levels of serum APOA-IV in GO patients were significantly higher than those in GD patients and controls. Specific proteins showed elevated expression in tear exosomes from GO patients, indicating that they may play important roles in GO pathogenesis.
Keywords: APOA-IV; Caspase-3; Graves’ ophthalmopathy; biomarker; exosome; tear.
Copyright © 2022 Shi, Zhao, Xin, Hou, Wang, Xie, Li and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Subekti I, Soewondo P, Soebardi S, Darmowidjojo B, Harbuwono DS, Purnamasari D, et al. . Practical guidelines management of graves ophthalmopathy. Acta Med Indones (2019) 51(4):364–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

