Disease activity, burden and suffering in patients with ulcerative colitis in the UK cohort recruited into the global ICONIC study
- PMID: 36561781
- PMCID: PMC9763636
- DOI: 10.1136/flgastro-2022-102104
Disease activity, burden and suffering in patients with ulcerative colitis in the UK cohort recruited into the global ICONIC study
Abstract
Objective: The Understanding the Impact of Ulcerative Colitis and Its Associated Disease Burden on Patients (ICONIC) was a 2-year, global, prospective, observational study assessing disease burden in adults recently diagnosed (≤36 months) with ulcerative colitis (UC) receiving routine outpatient care, irrespective of disease severity or treatment. A subanalysis was conducted to understand the UK perspective.
Design/method: All eligible consenting patients enrolled in ICONIC from the UK were included in the subanalysis of patient-reported and physician-reported outcomes at baseline and year 2 (Y2).
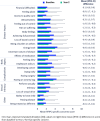
Results: Sixty-three UK patients were included (mean age 43.4 years, 58.7% female). At baseline and Y2, the mean (±SD) Simple Clinical Colitis Activity Index (SCCAI) scores were 3.6 (±3.3) and 1.5 (±1.5); Patient Modified Simple Clinical Colitis Activity Index (P-SSCAI) were 4.9 (±4.0) and 2.6 (±2.6), respectively. Physician-reported Pictorial Representation of Illness and Self Measure (PRISM) median scores (assessing inverse of suffering) were 3.5 (IQR 2.0-6.8) at baseline and 5.5 (IQR 3.6-6.9) at Y2; patient-reported PRISM scores were 4.7 (IQR 2.6-6.9) and 5.4 (IQR 3.2-8.0), respectively. At baseline, SCCAI and P-SCCAI were strongly correlated (r=0.86, p<0.0001), and patient-reported and physician-reported PRISM scores moderately correlated (r=0.67, p<0.0001). At Y2, moderate correlations were observed (SCCAI vs P-SCCAI: r=0.72, p<0.0001; patient-reported vs physician- reported PRISM: r=0.60, p<0.0001). Rating Form of IBD Patient Concerns scores indicated patients' greatest concerns were with energy level, having an ostomy bag and effects of medication (baseline scores >3.0).
Conclusions: These findings demonstrated the multifaceted burden of disease in patients recently diagnosed with UC in the UK. Agreement between patients and physicians on disease activity/severity varied according to the instrument used.
Keywords: ulcerative colitis.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: AH has served as consultant, advisory board member or speaker for AbbVie, Atlantic, Bristol-Myers Squibb, Celltrion, Falk, Ferring, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Shire and Takeda. She also serves on the Global Steering Committee for Genentech. DW has previously received support from AbbVie in the form of educational grants (attending congress) and honoraria for attending advisory boards. SL has no disclosures or conflicts of interest. SG: steering committees (AbbVie, Janssen and Celgene), consultancies (Takeda, Boehringer Ingelheim, Gilead, Eli Lilly and Roche), speaker commitments (AbbVie, Janssen, Celltrion, Takeda and Gilead). SVH and CH are employees of AbbVie. TA has received unrestricted grants for research, advisory board fees and support to attend conferences from AbbVie, Janssen, Merck, Celltrion, Celgene, Takeda, Ferring and Tillotts. CH is the author acting as guarantor for this article.
Figures
References
LinkOut - more resources
Full Text Sources