Alarming impact of the excessive use of tert-butylhydroquinone in food products: A narrative review
- PMID: 36561954
- PMCID: PMC9764193
- DOI: 10.1016/j.toxrep.2022.04.027
Alarming impact of the excessive use of tert-butylhydroquinone in food products: A narrative review
Abstract
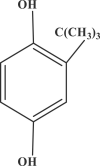
Tert-butyl hydroquinone (TBHQ) is a food additive commonly used as a more effective protectant in the food, cosmetic and pharmaceutical industries. However, the long-term exposure to TBHQ at higher doses (0.7 mg/kg) results in substantial danger to public health and brings a series of side effects, including cytotoxic, genotoxic, carcinogenic, and mutagenic effects. As a result, the global burden of chronic diseases has fascinated consumers and governments regarding the safety assessment of food additives. Regarding contradictory reports of various research about the application of food additives, the accurate monitoring of food additives is urgent. Notwithstanding, there are reports of the therapeutic effects of TBHQ under pathologic conditions through activation of nuclear factor erythroid 2-related factor 2. Thus, further investigations are required to investigate the impact of TBHQ on public health and evaluate its mechanism of action on various organs and cells. Therefore, this review aimed to investigate TBHQ safety through an overview of its impacts on different tissues, cells, and biological macromolecules as well as its therapeutic effects under pathologic conditions.
Keywords: Adverse impact; Food additives; Food safety; Overdose; Tert-butylhydroquinone.
© 2022 Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
NTP Toxicology and Carcinogenesis Studies of t-Butylhydroquinone (CAS No. 1948-33-0) in F344/N Rats and B6C3F(1) Mice (Feed Studies).Natl Toxicol Program Tech Rep Ser. 1997 May;459:1-326. Natl Toxicol Program Tech Rep Ser. 1997. PMID: 12587017
-
Cytotoxic and genotoxic effects of tert-butylhydroquinone, butylated hydroxyanisole and propyl gallate as synthetic food antioxidants.Food Sci Nutr. 2024 Aug 7;12(10):7004-7016. doi: 10.1002/fsn3.4373. eCollection 2024 Oct. Food Sci Nutr. 2024. PMID: 39479655 Free PMC article. Review.
-
A ratiometric electrochemical sensing strategy based on the self-assembly of Co NC/CNT and methylene blue for effective detection of the food additive tert-butylhydroquinone.Talanta. 2024 Jan 1;266(Pt 1):125024. doi: 10.1016/j.talanta.2023.125024. Epub 2023 Aug 2. Talanta. 2024. PMID: 37562227
-
Distribution of 2-tert-butyl-1,4-benzoquinone and its precursor, tert-butylhydroquinone, in typical edible oils and oleaginous foods marketed in Hangzhou City, China.Food Chem. 2021 Nov 1;361:130039. doi: 10.1016/j.foodchem.2021.130039. Epub 2021 May 11. Food Chem. 2021. PMID: 34022482
-
Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action.Food Chem. 2021 Aug 15;353:129488. doi: 10.1016/j.foodchem.2021.129488. Epub 2021 Mar 5. Food Chem. 2021. PMID: 33714793 Review.
Cited by
-
Competitive Foods' Nutritional Quality and Compliance with Smart Snacks Standards: An Analysis of a National Sample of U.S. Middle and High Schools.Nutrients. 2024 Jan 17;16(2):275. doi: 10.3390/nu16020275. Nutrients. 2024. PMID: 38257169 Free PMC article.
-
The Threat of Food Additive Occurrence in the Environment-A Case Study on the Example of Swimming Pools.Foods. 2023 Mar 11;12(6):1188. doi: 10.3390/foods12061188. Foods. 2023. PMID: 36981116 Free PMC article.
-
Comparative QSPR study of food preservatives using topological indices and regression models.Sci Rep. 2025 Jul 8;15(1):24557. doi: 10.1038/s41598-025-08002-5. Sci Rep. 2025. PMID: 40628862 Free PMC article.
-
In Vivo and In Silico Studies of the Hepatoprotective Activity of Tert-Butylhydroquinone.Int J Mol Sci. 2023 Dec 29;25(1):475. doi: 10.3390/ijms25010475. Int J Mol Sci. 2023. PMID: 38203648 Free PMC article.
-
Effect of using biodegradable film constituting red grape anthocyanins as a novel packaging on the qualitative attributes of emergency food bars during storage.Food Sci Nutr. 2024 Jan 25;12(4):2702-2723. doi: 10.1002/fsn3.3951. eCollection 2024 Apr. Food Sci Nutr. 2024. PMID: 38628210 Free PMC article.
References
-
- Akhtar‐Danesh N., et al. Parents’ perceptions and attitudes on childhood obesity: AQ‐methodology study. J. Am. Acad. Nurse Pract. 2011;23(2):67–75. - PubMed
-
- King T., et al. Food safety for food security: relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017;68:160–175.
-
- Constable A., et al. An integrated approach to the safety assessment of food additives in early life. Toxicol. Res. Appl. 2017;1 p. 2397847317707370.
-
- Pressman P., et al. Food additive safety: a review of toxicologic and regulatory issues. Toxicol. Res. Appl. 2017;1 p. 2397847317723572.
-
- Fathi F., et al. Kinetic and thermodynamic studies of bovine serum albumin interaction with ascorbyl palmitate and ascorbyl stearate food additives using surface plasmon resonance. Food Chem. 2018;246:228–232. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

