Small molecule-based immunomodulators for cancer therapy
- PMID: 36562003
- PMCID: PMC9764074
- DOI: 10.1016/j.apsb.2022.11.007
Small molecule-based immunomodulators for cancer therapy
Abstract
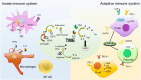
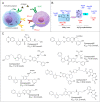
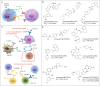
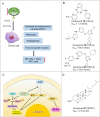
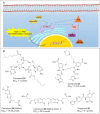
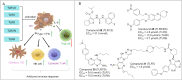
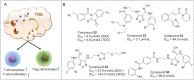
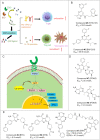
Immunotherapy has led to a paradigm shift in the treatment of cancer. Current cancer immunotherapies are mostly antibody-based, thus possessing advantages in regard to pharmacodynamics (e.g., specificity and efficacy). However, they have limitations in terms of pharmacokinetics including long half-lives, poor tissue/tumor penetration, and little/no oral bioavailability. In addition, therapeutic antibodies are immunogenic, thus may cause unwanted adverse effects. Therefore, researchers have shifted their efforts towards the development of small molecule-based cancer immunotherapy, as small molecules may overcome the above disadvantages associated with antibodies. Further, small molecule-based immunomodulators and therapeutic antibodies are complementary modalities for cancer treatment, and may be combined to elicit synergistic effects. Recent years have witnessed the rapid development of small molecule-based cancer immunotherapy. In this review, we describe the current progress in small molecule-based immunomodulators (inhibitors/agonists/degraders) for cancer therapy, including those targeting PD-1/PD-L1, chemokine receptors, stimulator of interferon genes (STING), Toll-like receptor (TLR), etc. The tumorigenesis mechanism of various targets and their respective modulators that have entered clinical trials are also summarized.
Keywords: Agonists; Cancer immunotherapy; Immunomodulators; Inhibitors; Small molecules.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures


References
-
- Chen S., Song Z., Zhang A. Small-molecule immuno-oncology therapy: advances, challenges and new directions. Curr Top Med Chem. 2019;19:180–185. - PubMed
-
- Lin X., Lu X., Luo G., Xiang H. Progress in PD-1/PD-l1 pathway inhibitors: from biomacromolecules to small molecules. Eur J Med Chem. 2020;186 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

