The G protein-coupled receptor ligand apelin-13 ameliorates skeletal muscle atrophy induced by chronic kidney disease
- PMID: 36562292
- PMCID: PMC9891924
- DOI: 10.1002/jcsm.13159
The G protein-coupled receptor ligand apelin-13 ameliorates skeletal muscle atrophy induced by chronic kidney disease
Abstract
Background: Targeting of the apelin-apelin receptor (Apj) system may serve as a useful therapeutic intervention for the management of chronic kidney disease (CKD)-induced skeletal muscle atrophy. We investigated the roles and efficacy of the apelin-Apj system in CKD-induced skeletal muscle atrophy.
Methods: The 5/6-nephrectomized mice were used as CKD models. AST-120, a charcoal adsorbent of uraemic toxins (8 w/w% in diet), or apelin (1 μmol/kg) was administered to CKD mice to investigate the mechanism and therapeutic potential of apelin on CKD-induced skeletal muscle atrophy. The effect of indoxyl sulfate, a uraemic toxin, or apelin on skeletal muscle atrophy was evaluated using mouse myoblast cells (C2C12 cells) in vitro.
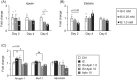
Results: Skeletal muscle atrophy developed over time following nephrectomy at 12 weeks, as confirmed by a significant increase of atrogin-1 and myostatin mRNA expression in the gastrocnemius (GA) muscle and a decrease of lower limb skeletal muscle weight (P < 0.05, 0.01 and 0.05, respectively). Apelin expression in GA muscle was significantly decreased (P < 0.05) and elabela, another Apj endogenous ligand, tended to show a non-significant decrease at 12 weeks after nephrectomy. Administration of AST-120 inhibited the decline of muscle weight and increase of atrogin-1 and myostatin expression. Apelin and elabela expression was slightly improved by AST-120 administration but Apj expression was not, suggesting the involvement of uraemic toxins in endogenous Apj ligand expression. The administration of apelin at 1.0 μmol/kg for 4 weeks to CKD mice suppressed the increase of atrogin-1 and myostatin, increased apelin and Apj mRNA expression at 30 min after apelin administration and significantly ameliorated weight loss and a decrease of the cross-sectional area of hindlimb skeletal muscle.
Conclusions: This study demonstrated for the first time the association of the Apj endogenous ligand-uraemic toxin axis with skeletal muscle atrophy in CKD and the utility of therapeutic targeting of the apelin-Apj system.
Keywords: Apj; apelin; chronic kidney disease; elabela; skeletal muscle atrophy; uraemic toxin.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors report no conflicts of interest.
Figures





Similar articles
-
Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1.Sci Rep. 2016 Aug 23;6:32084. doi: 10.1038/srep32084. Sci Rep. 2016. PMID: 27549031 Free PMC article.
-
Formononetin ameliorates muscle atrophy by regulating myostatin-mediated PI3K/Akt/FoxO3a pathway and satellite cell function in chronic kidney disease.J Cell Mol Med. 2021 Feb;25(3):1493-1506. doi: 10.1111/jcmm.16238. Epub 2021 Jan 6. J Cell Mol Med. 2021. PMID: 33405354 Free PMC article.
-
Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction.J Cachexia Sarcopenia Muscle. 2017 Oct;8(5):735-747. doi: 10.1002/jcsm.12202. Epub 2017 Jun 12. J Cachexia Sarcopenia Muscle. 2017. PMID: 28608457 Free PMC article.
-
Apelin/APJ system: A critical regulator of vascular smooth muscle cell.J Cell Physiol. 2018 Jul;233(7):5180-5188. doi: 10.1002/jcp.26339. Epub 2018 Jan 23. J Cell Physiol. 2018. PMID: 29215755 Review.
-
Apelin and Elabela/Toddler; double ligands for APJ/Apelin receptor in heart development, physiology, and pathology.Peptides. 2019 Jan;111:62-70. doi: 10.1016/j.peptides.2018.04.011. Epub 2018 Apr 21. Peptides. 2019. PMID: 29684595 Review.
Cited by
-
Beneficial effects of Apelin-13 on metabolic diseases and exercise.Front Endocrinol (Lausanne). 2023 Nov 28;14:1285788. doi: 10.3389/fendo.2023.1285788. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38089606 Free PMC article. Review.
-
Myokines: metabolic regulation in obesity and type 2 diabetes.Life Metab. 2024 Mar 2;3(3):loae006. doi: 10.1093/lifemeta/loae006. eCollection 2024 Jun. Life Metab. 2024. PMID: 39872377 Free PMC article. Review.
-
The Ability of AST-120 to Lower the Serum Indoxyl Sulfate Level Improves Renal Outcomes and the Lipid Profile in Diabetic and Nondiabetic Animal Models of Chronic Kidney Disease: A Meta-Analysis.Toxins (Basel). 2024 Dec 16;16(12):544. doi: 10.3390/toxins16120544. Toxins (Basel). 2024. PMID: 39728802 Free PMC article.
-
Skeletal Muscle Injury in Chronic Kidney Disease-From Histologic Changes to Molecular Mechanisms and to Novel Therapies.Int J Mol Sci. 2024 May 8;25(10):5117. doi: 10.3390/ijms25105117. Int J Mol Sci. 2024. PMID: 38791164 Free PMC article. Review.
-
How Can Promoting Skeletal Muscle Health and Exercise in Children and Adolescents Prevent Insulin Resistance and Type 2 Diabetes?Life (Basel). 2024 Sep 21;14(9):1198. doi: 10.3390/life14091198. Life (Basel). 2024. PMID: 39337980 Free PMC article. Review.
References
-
- Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimbürger O, Bárány P, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr 2008;27:557–564. - PubMed
-
- Zhou Y, Hellberg M, Svensson P, Höglund P, Clyne N. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3–5. Nephrol Dial Transplant 2018;33:342–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

