Zhizhu decoction alleviates slow transit constipation by regulating aryl hydrocarbon receptor through gut microbiota
- PMID: 36562308
- PMCID: PMC9793913
- DOI: 10.1080/13880209.2022.2157020
Zhizhu decoction alleviates slow transit constipation by regulating aryl hydrocarbon receptor through gut microbiota
Erratum in
-
Correction.Pharm Biol. 2023 Dec;61(1):1413. doi: 10.1080/13880209.2023.2254065. Pharm Biol. 2023. PMID: 37665171 Free PMC article. No abstract available.
Abstract
Context: Slow transit constipation (STC), the most common type of constipation, seriously affects the life of patients. Zhizhu decoction (ZZD), a traditional Chinese medicine compound, has is effective against functional constipation, but the mechanism is still unclear.
Objective: This research explores the mechanism of ZZD on STC from the perspective of metabolomics and gut microbiota.
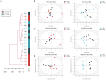
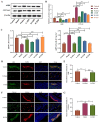
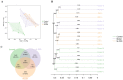
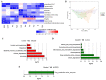
Materials and methods: Fifty-four C57BL/6 mice were randomly divided into six groups (n = 9): control (control); STC (model); positive control (positive); low-dose (5 g/kg; L-ZZD), medium-dose (10 g/kg; M-ZZD), and high-dose (20 g/kg; H-ZZD) ZZD treatment. Following treatment of mice with ZZD for two weeks, the changes in intestinal motility, colon histology, intestinal neurotransmitters, and aryl hydrocarbon receptor (AHR) pathway determined the effects of ZZD on the pathophysiology of STC. LC-MS targeting serum metabolomics was used to analyze the regulation of ZZD on neurotransmitters, and 16S rRNA high-throughput sequencing was used to detect the regulation of the gut microbiome.
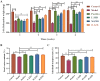
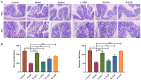
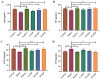
Results: ZZD had the highest content of naringin (6348.1 mg/L), and could significantly increase the 24 h defecations (1.10- to 1.42-fold), fecal moisture (1.14-fold) and intestinal transport rate (1.28-fold) of STC mice, increased the thickness of the mucosal and muscular tissue (1.18- to 2.16-fold) and regulated the neurotransmitters in the colon of STC mice. Moreover, ZZD significantly activated the AHR signaling pathway, and also affected the composition of gut microbiota in STC mice.
Discussion and conclusions: The beneficial effect and the possible mechanism of ZZD on STC could provide a theoretical basis for the broader clinical application of ZZD.
Keywords: 16S rRNA sequencing; Intestinal neurotransmitters; functional constipation; intestinal motility; metabolomics.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Zhizhu Decoction Alleviates Intestinal Barrier Damage via Regulating SIRT1/FoxO1 Signaling Pathway in Slow Transit Constipation Model Mice.Chin J Integr Med. 2023 Sep;29(9):809-817. doi: 10.1007/s11655-022-3539-2. Epub 2022 Aug 31. Chin J Integr Med. 2023. PMID: 36044116
-
Analysis of Gut Microbiome and Metabolite Characteristics in Patients with Slow Transit Constipation.Dig Dis Sci. 2021 Sep;66(9):3026-3035. doi: 10.1007/s10620-020-06500-2. Epub 2020 Aug 7. Dig Dis Sci. 2021. PMID: 32767153
-
Correlation between slow transit constipation and spleen deficiency, and gut microbiota: a pilot study.J Tradit Chin Med. 2022 Jun;42(3):353-363. doi: 10.19852/j.cnki.jtcm.20220408.002. J Tradit Chin Med. 2022. PMID: 35610004 Free PMC article.
-
[Research progress of mechanisms of acupuncture in treating slow transit constipation].Zhen Ci Yan Jiu. 2023 Apr 25;48(4):411-4. doi: 10.13702/j.1000-0607.20211387. Zhen Ci Yan Jiu. 2023. PMID: 37186208 Review. Chinese.
-
Research progress in the treatment of slow transit constipation by traditional Chinese medicine.J Ethnopharmacol. 2022 May 23;290:115075. doi: 10.1016/j.jep.2022.115075. Epub 2022 Feb 5. J Ethnopharmacol. 2022. PMID: 35134487 Review.
Cited by
-
Ameliorative effects of the mixed aqueous extract of Aurantii Fructus Immaturus and Magnoliae Officinalis Cortex on loperamide-induced STC mice.Heliyon. 2024 Jun 27;10(13):e33705. doi: 10.1016/j.heliyon.2024.e33705. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39040398 Free PMC article.
-
Barleriside A, an aryl hydrocarbon receptor antagonist, ameliorates podocyte injury through inhibiting oxidative stress and inflammation.Front Pharmacol. 2024 Aug 22;15:1386604. doi: 10.3389/fphar.2024.1386604. eCollection 2024. Front Pharmacol. 2024. PMID: 39239643 Free PMC article.
-
Single-cell transcriptomics reveals the interaction between fibroblasts and activated immune cells: an exploratory bioinformatics study of pro-inflammatory mechanisms in slow transit constipation.Int J Surg. 2025 Jun 1;111(6):3767-3780. doi: 10.1097/JS9.0000000000002415. Epub 2025 Apr 22. Int J Surg. 2025. PMID: 40265474 Free PMC article.
-
Activation of the aryl hydrocarbon receptor in inflammatory bowel disease: insights from gut microbiota.Front Cell Infect Microbiol. 2023 Oct 24;13:1279172. doi: 10.3389/fcimb.2023.1279172. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37942478 Free PMC article. Review.
-
Laxative effect of Zengye granule by modulating the SCF/c-Kit pathway and gut microbiota in constipated mice.Front Vet Sci. 2025 Jun 18;12:1628570. doi: 10.3389/fvets.2025.1628570. eCollection 2025. Front Vet Sci. 2025. PMID: 40607359 Free PMC article.
References
-
- Biancani P, Beinfeld MC, Coy DH, Hillemeier C, Walsh JH, Behar J.. 1988. Dysfunction of the gastrointestinal tract. Vasoactive intestinal peptide in peristalsis and sphincter function. Ann N Y Acad Sci. 527:546–567. - PubMed
-
- Black CJ, Ford AC.. 2018. Chronic idiopathic constipation in adults: epidemiology, pathophysiology, diagnosis and clinical management. Med J Aust. 209(2):86–91. - PubMed
-
- Camilleri M, Ford AC, Mawe GM, Dinning PG, Rao SS, Chey WD, Simrén M, Lembo A, Young-Fadok TM, Chang L.. 2017. Chronic constipation. Nat Rev Dis Primers. 3:17095. - PubMed
-
- Cervantes-Barragan L, Colonna M.. 2018. AHR signaling in the development and function of intestinal immune cells and beyond. Semin Immunopathol. 40(4):371–377. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
