Combined inhibition of ACLY and CDK4/6 reduces cancer cell growth and invasion
- PMID: 36562384
- PMCID: PMC9827262
- DOI: 10.3892/or.2022.8469
Combined inhibition of ACLY and CDK4/6 reduces cancer cell growth and invasion
Abstract
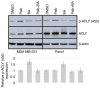
The use of small molecule kinase inhibitors, which target specific enzymes that are overactive in cancer cells, has revolutionized cancer patient treatment. To treat some types of breast cancer, CDK4/6 inhibitors, such as palbociclib, have been developed that target the phosphorylation of the retinoblastoma tumor suppressor gene. Acquired resistance to CDK4/6 inhibitors may be due to activation of the AKT pro‑survival signaling pathway that stimulates several processes, such as growth, metastasis and changes in metabolism that support rapid cell proliferation. The aim of the present study was to investigate whether targeting ATP citrate lyase (ACLY), a downstream target of AKT, may combine with CDK4/6 inhibition to inhibit tumorigenesis. The present study determined that ACLY is activated in breast and pancreatic cancer cells in response to palbociclib treatment and AKT mediates this effect. Inhibition of ACLY using bempedoic acid used in combination with palbociclib reduced cell viability in a panel of breast and pancreatic cancer cell lines. This effect was also observed using breast cancer cells grown in 3D cell culture. Mechanistically, palbociclib inhibited cell proliferation, whereas bempedoic acid stimulated apoptosis. Finally, using Transwell invasion assays and immunoblotting, the present study demonstrated that ACLY inhibition blocked cell invasion, when used alone or in combination with palbociclib. These data may yield useful information that could guide the development of future therapies aimed at the reduction of acquired resistance observed clinically.
Keywords: ATP citrate lyase; CDK4/6; bempedoic acid; palbociclib; resistance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- McCartney A, Migliaccio I, Bonechi M, Biagioni C, Romagnoli D, De Luca F, Galardi F, Risi E, De Santo I, Benelli M, et al. Mechanisms of resistance to CDK4/6 inhibitors: Potential implications and biomarkers for clinical practice. Front Oncol. 2019;9:666. doi: 10.3389/fonc.2019.00666. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

