Impact of BNT162b2 mRNA anti-SARS-CoV-2 vaccine on interferon-alpha production by plasmacytoid dendritic cells and autoreactive T cells in patients with systemic lupus erythematosus: The COVALUS project
- PMID: 36563528
- PMCID: PMC9760608
- DOI: 10.1016/j.jaut.2022.102987
Impact of BNT162b2 mRNA anti-SARS-CoV-2 vaccine on interferon-alpha production by plasmacytoid dendritic cells and autoreactive T cells in patients with systemic lupus erythematosus: The COVALUS project
Abstract
Objective: To evaluate the specific response of SLE patients to BNT162b2 vaccination and its impact on autoimmunity defined as in vivo production of interferon-alpha (IFNα) by plasmacytoid dendritic cells (pDCs) and autoreactive immune responses.
Methods: Our prospective study included SLE patients and healthy volunteers (HV) who received 2 doses of BNT162b2 vaccine 4 weeks apart. Subjects under immunosuppressive drugs or with evidence of prior COVID-19 were excluded. IgG anti-Spike SARS-CoV-2 (anti-S) antibodies, anti-S specific-B cells, anti-S specific T cells, in vivo INF-α production by pDCs, activation marker expression by pDCs and autoreactive anti-nuclear T cells were quantified before first injection, before second injection, and 3 and 6 months after first injection.
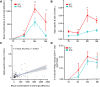
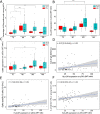
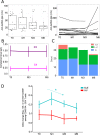
Results: Vaccinated SLE patients produced significantly lower IgG antibodies and specific B cells against SARS-CoV-2 as compared to HV. In contrast, anti-S T cell response did not significantly differ between SLE patients and HV. Following vaccination, the surface expression of HLA-DR and CD86 and the in vivo production of IFNα by pDCs significantly increased in SLE patients. The boosted expression of HLA-DR on pDCs induced by BNT162b2 vaccine correlated with the overall immune responses against SARS-CoV-2 (anti-S antibodies: r = 0.27 [0.05-0.46], p = 0.02; anti-S B cells: r = 0.19 [-0.03-0.39], p = 0.09); anti-S T cells: r = 0.28 [0.05-0.47], p = 0.016). Eventually, anti-SARS-CoV-2 vaccination was associated with an overall decrease of autoreactive T cells (slope = - 0.00067, p = 0.015).
Conclusion: BNT162b2 vaccine induces a transient in vivo activation of pDCs in SLE that contributes to the immune responses against SARS-CoV-2. Unexpectedly BNT162b2 vaccine also dampens the pool of circulating autoreactive T cells, suggesting that vaccination may have a beneficial impact on SLE disease.
Keywords: Plasmacytoid dendritic cells; SARS-CoV-2; Systemic lupus erythematosus; Type-1 interferon; mRNA vaccine.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

