Restoring vision and rebuilding the retina by Müller glial cell reprogramming
- PMID: 36563542
- PMCID: PMC10783479
- DOI: 10.1016/j.scr.2022.103006
Restoring vision and rebuilding the retina by Müller glial cell reprogramming
Abstract
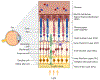
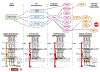
Müller glia are non-neuronal support cells that play a vital role in the homeostasis of the eye. Their radial-oriented processes span the width of the retina and respond to injury through a cellular response that can be detrimental or protective depending on the context. In some species, protective responses include the expression of stem cell-like genes which help to fuel new neuron formation and even restoration of vision. In many lower vertebrates including fish and amphibians, this response is well documented, however, in mammals it is severely limited. The remarkable plasticity of cellular reprogramming in lower vertebrates has inspired studies in mammals for repairing the retina and restoring sight, and recent studies suggest that mammals are also capable of regeneration, albeit to a lesser degree. Endogenous regeneration, whereby new retinal neurons are created from existing support cells, offers an exciting alternative approach to existing tissue transplant, gene therapy, and neural prosthetic approaches being explored in parallel. This review will highlight the role of Müller glia during retinal injury and repair. In the end, prospects for advancing retinal regeneration research will be considered.
Keywords: Cellular reprogramming; Müller glia; Retinal regeneration.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Daiger SP, Sullivan LS, Bowne SJ, Rossiter BJF, 1996. RetNet - Retinal Information Network. The University of Texas Health Science Center.

