Histopathologic and proteogenomic heterogeneity reveals features of clear cell renal cell carcinoma aggressiveness
- PMID: 36563681
- PMCID: PMC9839644
- DOI: 10.1016/j.ccell.2022.12.001
Histopathologic and proteogenomic heterogeneity reveals features of clear cell renal cell carcinoma aggressiveness
Abstract
Clear cell renal cell carcinomas (ccRCCs) represent ∼75% of RCC cases and account for most RCC-associated deaths. Inter- and intratumoral heterogeneity (ITH) results in varying prognosis and treatment outcomes. To obtain the most comprehensive profile of ccRCC, we perform integrative histopathologic, proteogenomic, and metabolomic analyses on 305 ccRCC tumor segments and 166 paired adjacent normal tissues from 213 cases. Combining histologic and molecular profiles reveals ITH in 90% of ccRCCs, with 50% demonstrating immune signature heterogeneity. High tumor grade, along with BAP1 mutation, genome instability, increased hypermethylation, and a specific protein glycosylation signature define a high-risk disease subset, where UCHL1 expression displays prognostic value. Single-nuclei RNA sequencing of the adverse sarcomatoid and rhabdoid phenotypes uncover gene signatures and potential insights into tumor evolution. In vitro cell line studies confirm the potential of inhibiting identified phosphoproteome targets. This study molecularly stratifies aggressive histopathologic subtypes that may inform more effective treatment strategies.
Keywords: CPTAC; UCHL1; clear cell renal cell carcinoma; glycoproteomics; histology; metabolome; phosphoproteomics; proteogenomics; single-nuclei RNA-seq; tumor heterogeneity.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



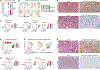
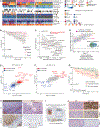



References
Publication types
MeSH terms
Substances
Grants and funding
- U24 CA210954/CA/NCI NIH HHS/United States
- U01 CA214116/CA/NCI NIH HHS/United States
- U24 CA210985/CA/NCI NIH HHS/United States
- U24 CA210967/CA/NCI NIH HHS/United States
- U24 CA210986/CA/NCI NIH HHS/United States
- U24 CA270823/CA/NCI NIH HHS/United States
- U24 CA210972/CA/NCI NIH HHS/United States
- U24 CA210955/CA/NCI NIH HHS/United States
- P30 ES017885/ES/NIEHS NIH HHS/United States
- U24 CA210993/CA/NCI NIH HHS/United States
- U24 CA210979/CA/NCI NIH HHS/United States
- U24 CA271114/CA/NCI NIH HHS/United States
- U24 CA271079/CA/NCI NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- U24 CA271037/CA/NCI NIH HHS/United States
- U24 CA271012/CA/NCI NIH HHS/United States
- U01 CA214114/CA/NCI NIH HHS/United States
- U01 CA214125/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

