Proximity proteomics identifies septins and PAK2 as decisive regulators of actomyosin-mediated expulsion of von Willebrand factor
- PMID: 36564030
- PMCID: PMC10023740
- DOI: 10.1182/blood.2022017419
Proximity proteomics identifies septins and PAK2 as decisive regulators of actomyosin-mediated expulsion of von Willebrand factor
Abstract
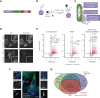
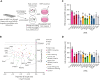
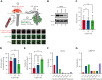
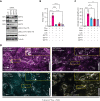
In response to tissue injury, within seconds the ultra-large glycoprotein von Willebrand factor (VWF) is released from endothelial storage organelles (Weibel-Palade bodies) into the lumen of the blood vasculature, where it leads to the recruitment of platelets. The marked size of VWF multimers represents an unprecedented burden on the secretory machinery of endothelial cells (ECs). ECs have evolved mechanisms to overcome this, most notably an actomyosin ring that forms, contracts, and squeezes out its unwieldy cargo. Inhibiting the formation or function of these structures represents a novel therapeutic target for thrombotic pathologies, although characterizing proteins associated with such a dynamic process has been challenging. We have combined APEX2 proximity labeling with an innovative dual loss-of-function screen to identify proteins associated with actomyosin ring function. We show that p21 activated kinase 2 (PAK2) recruits septin hetero-oligomers, a molecular interaction that forms a ring around exocytic sites. This cascade of events controls actomyosin ring function, aiding efficient exocytic release. Genetic or pharmacological inhibition of PAK2 or septins led to inefficient release of VWF and a failure to form platelet-catching strings. This new molecular mechanism offers additional therapeutic targets for the control of thrombotic disease and is highly relevant to other secretory systems that employ exocytic actomyosin machinery.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





Comment in
-
CSI: Weibel-Palade bodies.Blood. 2023 Feb 23;141(8):820-821. doi: 10.1182/blood.2022019268. Blood. 2023. PMID: 36821184 No abstract available.
References
-
- McCormack JJ, Lopes da Silva M, Ferraro F, Patella F, Cutler DF. Weibel-Palade bodies at a glance. J Cell Sci. 2017;130(21):3611–3617. - PubMed
-
- De Ceunynck K, De Meyer SF, Vanhoorelbeke K. Unwinding the von Willebrand factor strings puzzle. Blood. 2013;121(2):270–277. - PubMed
-
- Leebeek FW, Eikenboom JC. Von Willebrand's Disease. N Engl J Med. 2016;375(21):2067–2080. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

