Prevention of acute GVHD using an orthogonal IL-2/IL-2Rβ system to selectively expand regulatory T cells in vivo
- PMID: 36564052
- PMCID: PMC10082364
- DOI: 10.1182/blood.2022018440
Prevention of acute GVHD using an orthogonal IL-2/IL-2Rβ system to selectively expand regulatory T cells in vivo
Abstract
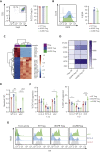
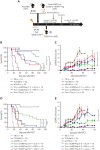
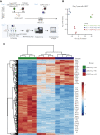
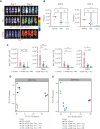
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative option for patients with hematological disorders and bone marrow (BM) failure syndromes. Graft-versus-host disease (GVHD) remains a leading cause of morbidity posttransplant. Regulatory T cell (Treg) therapies are efficacious in ameliorating GVHD but limited by variable suppressive capacities and the need for a high therapeutic dose. Here, we sought to expand Treg in vivo by expressing an orthogonal interleukin 2 receptor β (oIL-2Rβ) that would selectively interact with oIL-2 cytokine and not wild-type (WT) IL-2. To test whether the orthogonal system would preferentially drive donor Treg expansion, we used a murine major histocompatibility complex-disparate GVHD model of lethally irradiated BALB/c mice given T cell-depleted BM from C57BL/6 (B6) mice alone or together with B6Foxp3+GFP+ Treg or oIL-2Rβ-transduced Treg at low cell numbers that typically do not control GVHD with WT Treg. On day 2, B6 activated T cells (Tcons) were injected to induce GVHD. Recipients were treated with phosphate-buffered saline (PBS) or oIL-2 daily for 14 days, then 3 times weekly for an additional 14 days. Mice treated with oIL-2Rβ Treg and oIL-2 compared with those treated with PBS had enhanced GVHD survival, in vivo selective expansion of Tregs, and greater suppression of Tcon expansion in secondary lymphoid organs and intestines. Importantly, oIL-2Rβ Treg maintained graft-versus-tumor (GVT) responses in 2 distinct tumor models (A20 and MLL-AF9). These data demonstrate a novel approach to enhance the efficacy of Treg therapy in allo-HSCT using an oIL-2/oIL-2Rβ system that allows for selective in vivo expansion of Treg leading to GVHD protection and GVT maintenance.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: K.C.G. is the founder of Synthekine Therapeutics, which has licensed the ortho-2 technology. K.C.G. and L.K.P. are inventors on a patent application describing the ortho-2 system (biologically relevant orthogonal cytokine/receptor pairs, US patent no. 10,869,887B2). K.C.G., L.S., and L.K.P. are shareholders of Synthekine, a biotechnology company that has licensed the ortho–IL-2 technology. J.E.W. receives remuneration as an advisor to Magenta Therapeutics and BlueRock Therapeutics. B.R.B. receives remuneration as an advisor to Magenta Therapeutics and BlueRock Therapeutics; research funding from Carisma Therapeutics, Inc; and is a cofounder of Tmunity Therapeutics. The remaining authors declare no competing financial interests.
Figures





Comment in
-
GVHD prophylaxis: use an ortho IL-2/IL-2Rβ Treg system!Blood. 2023 Mar 16;141(11):1246-1247. doi: 10.1182/blood.2023019711. Blood. 2023. PMID: 36929438 No abstract available.
References
-
- Lucca LE, Dominguez-Villar M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat Rev Immunol. 2020;20(11):680–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F30 HL156312/HL/NHLBI NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States
- R01 HL118979/HL/NHLBI NIH HHS/United States
- R37 AI051321/AI/NIAID NIH HHS/United States
- R37 AI034495/AI/NIAID NIH HHS/United States
- R01 HL155114/HL/NHLBI NIH HHS/United States
- P01 HL075462/HL/NHLBI NIH HHS/United States
- R01 AR063070/AR/NIAMS NIH HHS/United States
- P01 HL158505/HL/NHLBI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- T32 AI007313/AI/NIAID NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- S10 RR027431/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

