Efficacy of guselkumab, a selective IL-23 inhibitor, in Preventing Arthritis in a Multicentre Psoriasis At-Risk cohort (PAMPA): protocol of a randomised, double-blind, placebo controlled multicentre trial
- PMID: 36564123
- PMCID: PMC9791418
- DOI: 10.1136/bmjopen-2022-063650
Efficacy of guselkumab, a selective IL-23 inhibitor, in Preventing Arthritis in a Multicentre Psoriasis At-Risk cohort (PAMPA): protocol of a randomised, double-blind, placebo controlled multicentre trial
Erratum in
-
Correction: Efficacy of guselkumab, a selective IL-23 inhibitor, in Preventing Arthritis in a Multicentre Psoriasis At-Risk cohort (PAMPA): protocol of a randomised, double-blind, placebo controlled multicentre trial.BMJ Open. 2023 Apr 6;13(4):e063650corr1. doi: 10.1136/bmjopen-2022-063650corr1. BMJ Open. 2023. PMID: 37024259 Free PMC article. No abstract available.
Abstract
Introduction: Psoriatic arthritis (PsA) is a complex, immune-mediated disease associated with skin psoriasis that, if left untreated, can lead to joint destruction. Up to 30% of patients with psoriasis progress to PsA. In most cases, psoriasis precedes synovio-entheseal inflammation by an average of 5-7 years, providing a unique opportunity for early and potentially preventive intervention in a susceptible and identifiable population. Guselkumab is an effective IL-23p19 inhibitor Food and Drug Administration (FDA-approved for treatment of moderate-to-severe psoriasis and PsA. The Preventing Arthritis in a Multicentre Psoriasis At-Risk cohort (PAMPA) study aims to evaluate the efficacy of guselkumab in preventing PsA and decreasing musculoskeletal power Doppler ultrasound (PDUS) abnormalities in a population of patients with psoriasis who are at-increased risk for PsA progression.
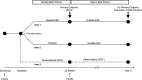
Methods and analysis: The PAMPA study is a multicentre, randomised, double-blind, placebo-controlled, interventional, preventive trial comparing PDUS involvement and conversion to PsA in patients with psoriasis at-increased risk for progression treated with guselkumab compared with non-biological standard of care. The study includes a screening period, a double-blind treatment period (24 weeks) and an open-label follow-up period (72 weeks). At baseline, 200 subjects will be randomised (1:1) to receive either guselkumab 100 mg (arm 1) or placebo switching to guselkumab 100 mg starting at week 24 (arm 2). Arm 3 will follow 150 at-risk psoriasis patients who decline biological therapy and randomisation. Changes from baseline in the PDUS score at week 24 and the difference in proportion of patients transitioning to PsA at 96 weeks will be examined as the coprimary endpoints.
Ethics and dissemination: Ethics approval for this study was granted by the coordinating centre's (NYU School of Medicine) Institutional Review Board (IRB). Each participating site received approval through their own IRBs. The findings will be shared in peer-reviewed articles and scientific conference presentations.
Trial registration number: NCT05004727.
Keywords: PREVENTIVE MEDICINE; Psoriasis; Rheumatology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KAM, SC, ZU, RBB, JS, JH, RC, KQ, FT have nothing to disclose. RHH has served as a consultant for Janssen. MT has served as a consultant for Horizon. VP has received honoraria for speaker and/or advisory board member roles from AbbVie, Almirall, Celgene, Janssen, Kyowa Kirin, LEO Pharma, Novartis, Pfizer, Sanofi, UCB and Union Therapeutics. In his role as Department Division Director of Dermatology at the University of Toronto. VP has received departmental support in the form of unrestricted educational grants from AbbVie, Bausch Health, Celgene, Janssen, LEO Pharma, Lilly, L’Oréal, NAOS, Novartis, Pfizer, Pierre-Fabre, Sandoz and Sanofi in the past 36 months. JY has served as a speaker, consultant, honoraria, and/or trialist for Abbvie, Amgen, Anacor, Astellas, Bausche, Baxalta, Boehringer Ingelheim, Celgene, Centocor, Coherus, Dermira, Eli Lilly, Forward, Galderma, Janssen, Leo, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sun Pharma, Takeda, UCB, Xenon. ALN declares that she has served as a consultant for Janssen, UCB, AbbVie, BMS and her immediate family member owns shares of stock in J&J, Eli Lilly, AbbVie and Pfizer. WG has received grants and research support from AbbVie, Amgen, Eli Lilly, Novartis, Pfizer and honorari for ad boards, invited talks, or consultation from AbbVie, Actelion, Amgen, Arylide, Bausch Health, Boehringer, Celgene, Cipher, Eli Lilly, Galderma, Janssen, LEO Pharma, Merck, Novartis, PeerVoice, Pfizer, Sanofi-Genzyme, Tribute, UCB, Valeant. Other: Clinical trials (study fees): AbbVie, Asana Biosciences, Astellas, Boerhinger-Ingleheim, Celgene, Corrona/National Psoriasis Foundation, Devonian, Eli Lilly, Galapagos, Galderma, Janssen, LEO Pharma, Novartis, Pfizer, Regeneron, UCB. RGT has served as a consultant for Novartis, Bioclinica. JFM has served as a consultant and/or investigator for Amgen, Bristol-Myers Squibb, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Sanofi, Regeneron, Sun Pharma, Biogen, Pfizer and Leo Pharma. AO has received esearch grants from AbbVie, Novartis, and Pfizer to University of Pennsylvania and Amgen to FORWARD/NDB; has research collaborations with GSK and Harvard Pilgrim; and has received consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB; royalties from Novartis (to spouse).PR has received esearch grants from Janssen and Novartis and speaker and consulting fees from Abbott, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, and Pfizer. LE has received grants from Novartis, Eli Lily, UCB, Pfizer, Abbvie, Sandoz, Janssen and consulting fees from Janssen, Abbvie, Pfizer, UCB, Eli Lily, Novartis. CTR has served as a consultant for Abbvie, Amgen, UCB, Novartis, Lilly, Janssen, MoonLake, Pfizer. JUS has served as a consultant for Janssen, Novartis, Pfizer, Sanofi, Amgen, UCB and AbbVie; and has received funding for investigator-initiated studies from Janssen and Pfizer. CG and SDC are employees of Janssen Scientific Affairs, LLC and shareholders in Johnson & Johnson, of which Janssen Scientific Affairs, LLC is a wholly owned subsidiary.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous