Exploiting the immunogenic potential of standard of care radiation or cisplatin therapy in preclinical models of HPV-associated malignancies
- PMID: 36564129
- PMCID: PMC9791467
- DOI: 10.1136/jitc-2022-005752
Exploiting the immunogenic potential of standard of care radiation or cisplatin therapy in preclinical models of HPV-associated malignancies
Abstract
Background: While radiation and chemotherapy are primarily purposed for their cytotoxic effects, a growing body of preclinical and clinical evidence demonstrates an immunogenic potential for these standard therapies. Accordingly, we sought to characterize the immunogenic potential of radiation and cisplatin in human tumor models of HPV-associated malignancies. These studies may inform rational combination immuno-oncology (IO) strategies to be employed in the clinic on the backbone of standard of care, and in so doing exploit the immunogenic potential of standard of care to improve durable responses in HPV-associated malignancies.
Methods: Retroviral transduction with HPV16 E7 established a novel HPV-associated sinonasal squamous cell carcinoma (SNSCC) cell line. Three established HPV16-positive cell lines were also studied (cervical carcinoma and head and neck squamous cell carcinoma). Following determination of sensitivities to standard therapies using MTT assays, flow cytometry was used to characterize induction of immunogenic cell stress following sublethal exposure to radiation or cisplatin, and the functional consequence of this induction was determined using impedance-based real time cell analysis cytotoxicity assays employing HPV16 E7-specific cytotoxic lymphocytes (CTLs) with or without N803 (IL-15/IL-15-Rα superagonist) or exogenous death receptor ligands. In vitro observations were translated using an in vivo xenograft NSG mouse model of human cervical carcinoma evaluating cisplatin in combination with CTL adoptive cell transfer.
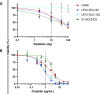
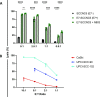
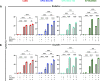
Results: We showed that subpopulations surviving clinically relevant doses of radiation or cisplatin therapy were more susceptible to CTL-mediated lysis in four of four tumor models of HPV-associated malignancies, serving as a model for HPV therapeutic vaccine or T-cell receptor adoptive cell transfer. This increased killing was further amplified by IL-15 agonism employing N803. We further characterized that radiation or cisplatin induced immunogenic cell stress in three of three cell lines, and consequently demonstrated that upregulated surface expression of Fas and TRAIL-R2 death receptors at least in part mediated enhanced CTL-mediated lysis. In vivo, cisplatin-induced immunogenic cell stress synergistically potentiated CTL-mediated tumor control in a human model of HPV-associated malignancy.
Conclusion: Standard of care radiation or cisplatin therapy induced immunogenic cell stress in preclinical models of HPV-associated malignancies, presenting an opportunity poised for exploitation by employing IO strategies in combination with standard of care.
Keywords: Combined Modality Therapy; Immunotherapy, Adoptive; Radiotherapy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NRL receives research funding from Merck Sharp & Dohme, holds stock in Navigen Pharmaceuticals and was a consultant for Cooltech, none of which are relevant to the present manuscript. All other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
