Role of CD14+ monocyte-derived oxidised mitochondrial DNA in the inflammatory interferon type 1 signature in juvenile dermatomyositis
- PMID: 36564154
- PMCID: PMC10176342
- DOI: 10.1136/ard-2022-223469
Role of CD14+ monocyte-derived oxidised mitochondrial DNA in the inflammatory interferon type 1 signature in juvenile dermatomyositis
Abstract
Objectives: To define the host mechanisms contributing to the pathological interferon (IFN) type 1 signature in Juvenile dermatomyositis (JDM).
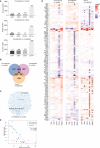
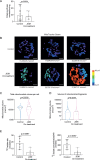
Methods: RNA-sequencing was performed on CD4+, CD8+, CD14+ and CD19+ cells sorted from pretreatment and on-treatment JDM (pretreatment n=10, on-treatment n=11) and age/sex-matched child healthy-control (CHC n=4) peripheral blood mononuclear cell (PBMC). Mitochondrial morphology and superoxide were assessed by fluorescence microscopy, cellular metabolism by 13C glucose uptake assays, and oxidised mitochondrial DNA (oxmtDNA) content by dot-blot. Healthy-control PBMC and JDM pretreatment PBMC were cultured with IFN-α, oxmtDNA, cGAS-inhibitor, TLR-9 antagonist and/or n-acetyl cysteine (NAC). IFN-stimulated gene (ISGs) expression was measured by qPCR. Total numbers of patient and controls for functional experiments, JDM n=82, total CHC n=35.
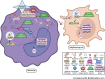
Results: Dysregulated mitochondrial-associated gene expression correlated with increased ISG expression in JDM CD14+ monocytes. Altered mitochondrial-associated gene expression was paralleled by altered mitochondrial biology, including 'megamitochondria', cellular metabolism and a decrease in gene expression of superoxide dismutase (SOD)1. This was associated with enhanced production of oxidised mitochondrial (oxmt)DNA. OxmtDNA induced ISG expression in healthy PBMC, which was blocked by targeting oxidative stress and intracellular nucleic acid sensing pathways. Complementary experiments showed that, under in vitro experimental conditions, targeting these pathways via the antioxidant drug NAC, TLR9 antagonist and to a lesser extent cGAS-inhibitor, suppressed ISG expression in pretreatment JDM PBMC.
Conclusions: These results describe a novel pathway where altered mitochondrial biology in JDM CD14+ monocytes lead to oxmtDNA production and stimulates ISG expression. Targeting this pathway has therapeutical potential in JDM and other IFN type 1-driven autoimmune diseases.
Keywords: Autoimmune Diseases; Dermatomyositis; Inflammation.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: LRW declares a consultancy from Pfizer unrelated to this study but in the field of this disease.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- 18DS03/DH_/Department of Health/United Kingdom
- 20164/VAC_/Versus Arthritis/United Kingdom
- 085860/WT_/Wellcome Trust/United Kingdom
- 22936/DH_/Department of Health/United Kingdom
- 21992/VAC_/Versus Arthritis/United Kingdom
- MRF-057-0001-RG-ROSS-C0797/MRF_/MRF_/United Kingdom
- 21593/VAC_/Versus Arthritis/United Kingdom
- 14518/VAC_/Versus Arthritis/United Kingdom
- MR/N003322/1/MRC_/Medical Research Council/United Kingdom
- GOSH BRC 18IR33/DH_/Department of Health/United Kingdom
- MRF-159-0006-ELP-MCDO-C0954/MRF_/MRF_/United Kingdom
- 21552/VAC_/Versus Arthritis/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

