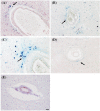
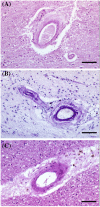
Arteriolar neuropathology in cerebral microvascular disease
- PMID: 36564356
- PMCID: PMC10350910
- DOI: 10.1111/nan.12875
Arteriolar neuropathology in cerebral microvascular disease
Abstract
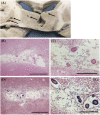
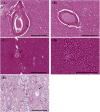
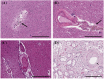
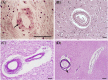
Cerebral microvascular disease (MVD) is an important cause of vascular cognitive impairment. MVD is heterogeneous in aetiology, ranging from universal ageing to the sporadic (hypertension, sporadic cerebral amyloid angiopathy [CAA] and chronic kidney disease) and the genetic (e.g., familial CAA, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy [CADASIL] and cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy [CARASIL]). The brain parenchymal consequences of MVD predominantly consist of lacunar infarcts (lacunes), microinfarcts, white matter disease of ageing and microhaemorrhages. MVD is characterised by substantial arteriolar neuropathology involving ubiquitous vascular smooth muscle cell (SMC) abnormalities. Cerebral MVD is characterised by a wide variety of arteriolar injuries but only a limited number of parenchymal manifestations. We reason that the cerebral arteriole plays a dominant role in the pathogenesis of each type of MVD. Perturbations in signalling and function (i.e., changes in proliferation, apoptosis, phenotypic switch and migration of SMC) are prominent in the pathogenesis of cerebral MVD, making 'cerebral angiomyopathy' an appropriate term to describe the spectrum of pathologic abnormalities. The evidence suggests that the cerebral arteriole acts as both source and mediator of parenchymal injury in MVD.
Keywords: cerebral angiomyopathy; cerebral arterioles; cerebral microvascular disease; neuropathology; smooth muscle cells.
© 2022 The Authors. Neuropathology and Applied Neurobiology published by John Wiley & Sons Ltd on behalf of British Neuropathological Society.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Figures