Molecular characterization of fungal endophthalmitis and keratitis caused by yeasts
- PMID: 36565720
- PMCID: PMC9825281
- DOI: 10.1093/mmy/myac099
Molecular characterization of fungal endophthalmitis and keratitis caused by yeasts
Abstract
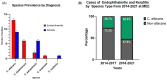
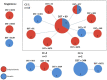
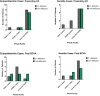
Candida species are the most common causes of sight-threatening fungal ocular infections in temperate regions of the world. Despite their relevance, little is known about the emergence of novel species and the molecular epidemiology of these infections. Here, we molecularly characterized 38 yeast isolates collected from patients diagnosed with endophthalmitis or keratitis at Massachusetts Eye and Ear from 2014 to 2021. Sequencing of the ITS1-5.8S-/ITS2 regions demonstrated that this population of yeasts was dominated by Candida spp. (37 out of 38; 97%), with 58% of the cases caused by C. albicans (n = 22) and the remaining by emerging non-albicans species, predominantly by C. parapsilosis (n = 8) and C. dubliniensis (n = 6). One isolate each was identified as C. tropicalis and Clavispora lusitaniae. Interestingly, all C. dubliniensis were isolated from endophthalmitis and most C. parapsilosis from keratitis. Multilocus sequence typing analysis of C. albicans showed a prevalence of CC-1 isolates that has DST69 as the putative founder, with 64% of them belonging to this clonal complex (CC). Isolates grouped within this cluster were more predominant in endophthalmitis (10 out of 14; 71%). One C. albicans CC-1 isolate was multi-azole resistant. In conclusion, we observed that nearly half of the ocular infections caused by yeasts are associated with C. albicans, with evidence for the emergence of non-albicans species that are differentially enriched in distinct ocular niches. Candida albicans isolates clustered within the predominant CC-1 group were particularly more common in endophthalmitis, demonstrating a potential pattern of ocular disease enrichment within this clade.
Keywords: Candida; Endophthalmitis; keratitis; molecular typing; ocular surface disease.
Plain language summary
Candida species are the most common pathogen responsible for ocular infections in the temperature regions of the world. Here, we sequenced and molecularly characterized the Candida species seen in patients who present to our hospital with infection to understand the species’ distribution over time.
© The Author(s) 2022. Published by Oxford University Press on behalf of The International Society for Human and Animal Mycology.
Figures
Similar articles
-
Analysis of global antifungal surveillance results reveals predominance of Erg11 Y132F alteration among azole-resistant Candida parapsilosis and Candida tropicalis and country-specific isolate dissemination.Int J Antimicrob Agents. 2020 Jan;55(1):105799. doi: 10.1016/j.ijantimicag.2019.09.003. Epub 2019 Sep 11. Int J Antimicrob Agents. 2020. PMID: 31520783
-
In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens.Am J Ophthalmol. 2004 May;137(5):820-5. doi: 10.1016/j.ajo.2003.11.078. Am J Ophthalmol. 2004. PMID: 15126145
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Candida Species From Eye Infections: Drug Susceptibility, Virulence Factors, and Molecular Characterization.Invest Ophthalmol Vis Sci. 2017 Aug 1;58(10):4201-4209. doi: 10.1167/iovs.17-22003. Invest Ophthalmol Vis Sci. 2017. PMID: 28837732
-
Resistance in human pathogenic yeasts and filamentous fungi: prevalence, underlying molecular mechanisms and link to the use of antifungals in humans and the environment.Dan Med J. 2016 Oct;63(10):B5288. Dan Med J. 2016. PMID: 27697142 Review.
Cited by
-
Candida Biofilm Eye Infection: Main Aspects and Advance in Novel Agents as Potential Source of Treatment.Antibiotics (Basel). 2023 Aug 2;12(8):1277. doi: 10.3390/antibiotics12081277. Antibiotics (Basel). 2023. PMID: 37627697 Free PMC article. Review.
-
Endophthalmitis patients in Makassar City: molecular identification of pathogenic fungal profile.BMC Infect Dis. 2024 Nov 19;24(1):1320. doi: 10.1186/s12879-024-10209-2. BMC Infect Dis. 2024. PMID: 39563233 Free PMC article.
-
Clinical Characteristics and In Vivo Confocal Microscopic Study in Candida Keratitis.Transl Vis Sci Technol. 2025 Jan 2;14(1):23. doi: 10.1167/tvst.14.1.23. Transl Vis Sci Technol. 2025. PMID: 39847374 Free PMC article.
References
-
- Das T, Agarwal M, Anand AR et al. Fungal endophthalmitis: analysis of 730 consecutive eyes from 7 tertiary eye care centers in India. Ophthalmol Retina. 2022; 6: 243–251. - PubMed
-
- Gopinathan U, Garg P, Fernandes M et al. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea. 2002; 21: 555–559. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

