One-year dynamics of antibody titers after three doses of SARS-CoV-2 BNT162b2 vaccine
- PMID: 36566162
- PMCID: PMC9767891
- DOI: 10.1016/j.vaccine.2022.12.042
One-year dynamics of antibody titers after three doses of SARS-CoV-2 BNT162b2 vaccine
Abstract
Background: A third dose of the BNT162b2 SARS-CoV-2 vaccine leads to a significant increase in antibody levels, however, concerns regarding the long-term persistence of this response exist. We assessed the humoral response for one year following vaccination.
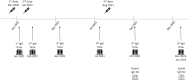
Methods: A prospective study among immunocompetent healthcare workers (HCW) who received three doses of BNT162b2. anti-spike antibody titers were measured at six predefined timepoints, from before the second vaccine dose, and up to one year afterwards, which is 4-6 months after the third dose. HCW with a history of SARS-CoV-2 infection were excluded.
Results: Seventy-six HCW had all the six serological measurements. Antibody titers significantly increased shortly following the third vaccine dose, and while declining, remained higher from all previous measurements for up to six months.
Conclusions: A third dose of BNT162b2 leads to a profound humoral response, which remains significantly higher than previous measurements, even after 6 months.
Keywords: COVID-19; Humoral response; Pfizer-BioNTech vaccine; SARS-CoV-2; Vaccination.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Krutikov M., Palmer T., Tut G., Fuller C., Azmi B., Giddings R., et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England. Lancet Healthy Longev. 2022;3(1):e13–e21. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

