Anti-C5a antibody vilobelimab treatment and the effect on biomarkers of inflammation and coagulation in patients with severe COVID-19: a substudy of the phase 2 PANAMO trial
- PMID: 36566174
- PMCID: PMC9789513
- DOI: 10.1186/s12931-022-02278-1
Anti-C5a antibody vilobelimab treatment and the effect on biomarkers of inflammation and coagulation in patients with severe COVID-19: a substudy of the phase 2 PANAMO trial
Abstract
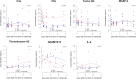
We recently reported in the phase 3 PANAMO trial that selectively blocking complement 5a (C5a) with vilobelimab led to improved survival in critically ill COVID-19 patients. C5a is an important contributor to the innate immune system and can also activate the coagulation system. High C5a levels have been reported in severely ill COVID-19 patients and correlate with disease severity and mortality. Previously, we assessed the potential benefit and safety of vilobelimab in severe COVID-19 patients. In the current substudy of the phase 2 PANAMO trial, we aim to explore the effects of vilobelimab on various biomarkers of inflammation and coagulation. Between March 31 and April 24, 2020, 17 patients with severe COVID-19 pneumonia were enrolled in an exploratory, open-label, randomised phase 2 trial. Blood markers of complement, endothelial activation, epithelial barrier disruption, inflammation, neutrophil activation, neutrophil extracellular trap (NET) formation and coagulopathy were measured using enzyme-linked immunosorbent assay (ELISA) or utilizing the Luminex platform. During the first 15 days after inclusion, change in biomarker concentrations between the two groups were modelled with linear mixed-effects models with spatial splines and compared. Eight patients were randomized to vilobelimab treatment plus best supportive care (BSC) and nine patients were randomized to BSC only. A significant decrease over time was seen in the vilobelimab plus BSC group for C5a compared to the BSC only group (p < 0.001). ADAMTS13 levels decreased over time in the BSC only group compared to the vilobelimab plus BSC group (p < 0.01) and interleukin-8 (IL-8) levels were statistically more suppressed in the vilobelimab plus BSC group compared to the BSC group (p = 0.03). Our preliminary results show that C5a inhibition decreases the inflammatory response and hypercoagulability, which likely explains the beneficial effect of vilobelimab in severe COVID-19 patients. Validation of these results in a larger sample size is warranted.
Keywords: C5a; COVID-19; Complement; Complement inhibition; SARS-CoV-2; Vilobelimab.
© 2022. The Author(s).
Conflict of interest statement
Prof. Dr. Vlaar reports personal fees from InflaRx paid to Amsterdam UMC, during conduct of the study. Dr. Habel and Dr. Xu are employees of InflaRx. All other authors declare no competing interests.
Figures
References
-
- Vlaar APJ, Witzenrath M, van Paassen P, Heunks LMA, Mourvillier B, de Bruin S, et al. Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;S2213–2600(22):00297–301. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

