Expanded in vivo substrate profile of the yeast N-terminal acetyltransferase NatC
- PMID: 36567016
- PMCID: PMC9867985
- DOI: 10.1016/j.jbc.2022.102824
Expanded in vivo substrate profile of the yeast N-terminal acetyltransferase NatC
Abstract
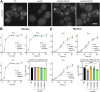
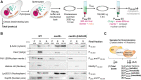
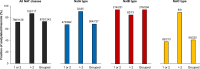
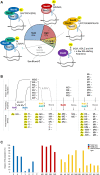
N-terminal acetylation is a conserved protein modification among eukaryotes. The yeast Saccharomyces cerevisiae is a valuable model system for studying this modification. The bulk of protein N-terminal acetylation in S. cerevisiae is catalyzed by the N-terminal acetyltransferases NatA, NatB, and NatC. Thus far, proteome-wide identification of the in vivo protein substrates of yeast NatA and NatB has been performed by N-terminomics. Here, we used S. cerevisiae deleted for the NatC catalytic subunit Naa30 and identified 57 yeast NatC substrates by N-terminal combined fractional diagonal chromatography analysis. Interestingly, in addition to the canonical N-termini starting with ML, MI, MF, and MW, yeast NatC substrates also included MY, MK, MM, MA, MV, and MS. However, for some of these substrate types, such as MY, MK, MV, and MS, we also uncovered (residual) non-NatC NAT activity, most likely due to the previously established redundancy between yeast NatC and NatE/Naa50. Thus, we have revealed a complex interplay between different NATs in targeting methionine-starting N-termini in yeast. Furthermore, our results showed that ectopic expression of human NAA30 rescued known NatC phenotypes in naa30Δ yeast, as well as partially restored the yeast NatC Nt-acetylome. Thus, we demonstrate an evolutionary conservation of NatC from yeast to human thereby underpinning future disease models to study pathogenic NAA30 variants. Overall, this work offers increased biochemical and functional insights into NatC-mediated N-terminal acetylation and provides a basis for future work to pinpoint the specific molecular mechanisms that link the lack of NatC-mediated N-terminal acetylation to phenotypes of NatC deletion yeast.
Keywords: N-alpha-acetyltransferase 30; N-terminal acetylation; N-terminal acetylome; S. cerevisiae disease model; glycerol metabolism; mitochondrial metabolism; non-fermentable sugar phenotype; protein modification; subcellular fractionation; virus assembly.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Aksnes H., Hole K., Arnesen T. Molecular, cellular, and physiological significance of N-terminal acetylation. Int. Rev. Cell Mol. Biol. 2015;316:267–305. - PubMed
-
- Aksnes H., Drazic A., Marie M., Arnesen T. First things first: vital protein marks by N-terminal acetyltransferases. Trends Biochem. Sci. 2016;41:746–760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

