Neonatal exposures to sevoflurane in rhesus monkeys alter synaptic ultrastructure in later life
- PMID: 36567715
- PMCID: PMC9772858
- DOI: 10.1016/j.isci.2022.105685
Neonatal exposures to sevoflurane in rhesus monkeys alter synaptic ultrastructure in later life
Abstract
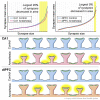
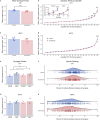
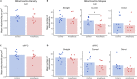
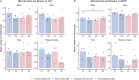
Repeated or prolonged early life exposure to anesthesia is neurotoxic in animals and associated with neurocognitive impairment in later life in humans. We used electron microscopy with unbiased stereological sampling to assess synaptic ultrastructure in dorsolateral prefrontal cortex (dlPFC) and hippocampal CA1 of female and male rhesus monkeys, four years after three 4-h exposures to sevoflurane during the first five postnatal weeks. This allowed us to ascertain long-term consequences of anesthesia exposure without confounding effects of surgery or illness. Synapse areas were reduced in the largest synapses in CA1 and dlPFC, predominantly in perforated spinous synapses in CA1 and nonperforated spinous synapses in dlPFC. Mitochondrial morphology and localization changed subtly in both areas. Synapse areas in CA1 correlated with response to a mild social stressor. Thus, exposure to anesthesia in infancy can cause long-term ultrastructural changes in primates, which may be substrates for long-term alterations in synaptic transmission and behavioral deficits.
Keywords: Cellular neuroscience; Developmental neuroscience.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ing C., Jackson W.M., Zaccariello M.J., Goldberg T.E., McCann M.-E., Grobler A., Davidson A., Sun L., Li G., Warner D.O. Prospectively assessed neurodevelopmental outcomes in studies of anaesthetic neurotoxicity in children: a systematic review and meta-analysis. Br. J. Anaesth. 2021;126:433–444. doi: 10.1016/j.bja.2020.10.022. - DOI - PMC - PubMed
-
- Ing C., DiMaggio C., Whitehouse A., Hegarty M.K., Brady J., von Ungern-Sternberg B.S., Davidson A., Wood A.J.J., Li G., Sun L.S. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–e485. doi: 10.1542/peds.2011-3822. - DOI - PubMed
-
- Ing C.H., DiMaggio C.J., Malacova E., Whitehouse A.J., Hegarty M.K., Feng T., Brady J.E., von Ungern-Sternberg B.S., Davidson A.J., Wall M.M., et al. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;120:1319–1332. doi: 10.1097/ALN.0000000000000248. - DOI - PubMed
-
- Wilder R.T., Flick R.P., Sprung J., Katusic S.K., Barbaresi W.J., Mickelson C., Gleich S.J., Schroeder D.R., Weaver A.L., Warner D.O. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

