Modelling skeletal pain harnessing tissue engineering
- PMID: 36567849
- PMCID: PMC9766883
- DOI: 10.1007/s44164-022-00028-7
Modelling skeletal pain harnessing tissue engineering
Abstract
Bone pain typically occurs immediately following skeletal damage with mechanical distortion or rupture of nociceptive fibres. The pain mechanism is also associated with chronic pain conditions where the healing process is impaired. Any load impacting on the area of the fractured bone will stimulate the nociceptive response, necessitating rapid clinical intervention to relieve pain associated with the bone damage and appropriate mitigation of any processes involved with the loss of bone mass, muscle, and mobility and to prevent death. The following review has examined the mechanisms of pain associated with trauma or cancer-related skeletal damage focusing on new approaches for the development of innovative therapeutic interventions. In particular, the review highlights tissue engineering approaches that offer considerable promise in the application of functional biomimetic fabrication of bone and nerve tissues. The strategic combination of bone and nerve tissue engineered models provides significant potential to develop a new class of in vitro platforms, capable of replacing in vivo models and testing the safety and efficacy of novel drug treatments aimed at the resolution of bone-associated pain. To date, the field of bone pain research has centred on animal models, with a paucity of data correlating to the human physiological response. This review explores the evident gap in pain drug development research and suggests a step change in approach to harness tissue engineering technologies to recapitulate the complex pathophysiological environment of the damaged bone tissue enabling evaluation of the associated pain-mimicking mechanism with significant therapeutic potential therein for improved patient quality of life.
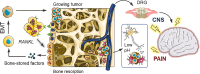
Graphical abstract: Rationale underlying novel drug testing platform development. Pain detected by the central nervous system and following bone fracture cannot be treated or exclusively alleviated using standardised methods. The pain mechanism and specificity/efficacy of pain reduction drugs remain poorly understood. In vivo and ex vivo models are not yet able to recapitulate the various pain events associated with skeletal damage. In vitro models are currently limited by their inability to fully mimic the complex physiological mechanisms at play between nervous and skeletal tissue and any disruption in pathological states. Robust innovative tissue engineering models are needed to better understand pain events and to investigate therapeutic regimes.
Keywords: Bone cancer; Bone pain; Fracture; In vitro models; Nerve.
© The Author(s) 2022.
Figures

References
-
- Alves CJ, Neto E, Sousa DM, et al. Fracture pain-traveling unknown pathways. Bone. 2016;85:107–14. 10.1016/j.bone.2016.01.026. - PubMed
-
- Lortet-Tieulent J, Georges D, Bray F, Vaccarella S. Profiling global cancer incidence and mortality by socioeconomic development. Int J Cancer. 2020;147:3029–36. 10.1002/ijc.33114. - PubMed
-
- Frost CØ, Hansen RR, Heegaard AM. Bone pain: current and future treatments. Curr Opin Pharmacol. 2016;28:31–7. 10.1016/j.coph.2016.02.007. - PubMed
-
- Ripamonti C, Fulfaro F. Malignant bone pain: pathophysiology and treatments. Curr Rev Pain. 2000;4:187–96. 10.1007/s11916-000-0078-3. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
