Tenuous transcriptional threshold of human sex determination. II. SRY exploits water-mediated clamp at the edge of ambiguity
- PMID: 36568077
- PMCID: PMC9771472
- DOI: 10.3389/fendo.2022.1029177
Tenuous transcriptional threshold of human sex determination. II. SRY exploits water-mediated clamp at the edge of ambiguity
Abstract
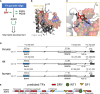
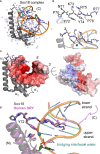
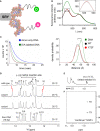
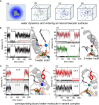
Y-encoded transcription factor SRY initiates male differentiation in therian mammals. This factor contains a high-mobility-group (HMG) box, which mediates sequence-specific DNA binding with sharp DNA bending. A companion article in this issue described sex-reversal mutations at box position 72 (residue 127 in human SRY), invariant as Tyr among mammalian orthologs. Although not contacting DNA, the aromatic ring seals the domain's minor wing at a solvent-exposed junction with a basic tail. A seeming paradox was posed by the native-like biochemical properties of inherited Swyer variant Y72F: its near-native gene-regulatory activity is consistent with the father's male development, but at odds with the daughter's XY female somatic phenotype. Surprisingly, aromatic rings (Y72, F72 or W72) confer higher transcriptional activity than do basic or polar side chains generally observed at solvated DNA interfaces (Arg, Lys, His or Gln). Whereas biophysical studies (time-resolved fluorescence resonance energy transfer and heteronuclear NMR spectroscopy) uncovered only subtle perturbations, dissociation of the Y72F complex was markedly accelerated relative to wild-type. Studies of protein-DNA solvation by molecular-dynamics (MD) simulations of an homologous high-resolution crystal structure (SOX18) suggest that Y72 para-OH anchors a network of water molecules at the tail-DNA interface, perturbed in the variant in association with nonlocal conformational fluctuations. Loss of the Y72 anchor among SRY variants presumably "unclamps" its basic tail, leading to (a) rapid DNA dissociation despite native affinity and (b) attenuated transcriptional activity at the edge of sexual ambiguity. Conservation of Y72 suggests that this water-mediated clamp operates generally among SRY and metazoan SOX domains.
Keywords: DNA dynamics; DNA intercalation; indirect readout; nucleic-acid recognition; protein hydration.
Copyright © 2022 Racca, Chatterjee, Chen, Rai, Yang, Georgiadis, Haas and Weiss.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Tenuous Transcriptional Threshold of Human Sex Determination. I. SRY and Swyer Syndrome at the Edge of Ambiguity.Front Endocrinol (Lausanne). 2022 Jul 26;13:945030. doi: 10.3389/fendo.2022.945030. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35957822 Free PMC article.
-
Structure-function relationships in human testis-determining factor SRY: an aromatic buttress underlies the specific DNA-bending surface of a high mobility group (HMG) box.J Biol Chem. 2014 Nov 21;289(47):32410-29. doi: 10.1074/jbc.M114.597526. Epub 2014 Sep 24. J Biol Chem. 2014. PMID: 25258310 Free PMC article.
-
SRY and human sex determination: the basic tail of the HMG box functions as a kinetic clamp to augment DNA bending.J Mol Biol. 2006 Apr 21;358(1):172-92. doi: 10.1016/j.jmb.2006.01.060. Epub 2006 Feb 6. J Mol Biol. 2006. PMID: 16504207
-
Sry, Sox9 and mammalian sex determination.EXS. 2001;(91):25-56. doi: 10.1007/978-3-0348-7781-7_3. EXS. 2001. PMID: 11301599 Review.
-
Molecular basis of mammalian sexual determination: activation of Müllerian inhibiting substance gene expression by SRY.Science. 1994 Dec 2;266(5190):1494-500. doi: 10.1126/science.7985018. Science. 1994. PMID: 7985018 Review.
Cited by
-
Tenuous Transcriptional Threshold of Human Sex Determination. I. SRY and Swyer Syndrome at the Edge of Ambiguity.Front Endocrinol (Lausanne). 2022 Jul 26;13:945030. doi: 10.3389/fendo.2022.945030. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35957822 Free PMC article.
-
Role of nucleobase-specific interactions in the binding and bending of DNA by human male sex determination factor SRY.J Biol Chem. 2024 Sep;300(9):107683. doi: 10.1016/j.jbc.2024.107683. Epub 2024 Aug 20. J Biol Chem. 2024. PMID: 39168182 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

