Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database
- PMID: 36568085
- PMCID: PMC9770009
- DOI: 10.3389/fendo.2022.1043789
Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database
Abstract
Objective: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have significantly improved clinical effects on glycemic control. However, real-world data concerning the difference in gastrointestinal adverse events (AEs) among different GLP-1 RAs are still lacking. Our study aimed to characterize and compare gastrointestinal AEs among different marketed GLP-1 RAs (exenatide, liraglutide, dulaglutide, lixisenatide, and semaglutide) based on real-world data.
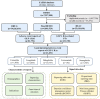
Methods: Disproportionality analysis was used to evaluate the association between GLP-1 RAs and gastrointestinal adverse events. Data were extracted from the US FDA Adverse Event Reporting System (FAERS) database between January 2018 and September 2022. Clinical characteristics, the time-to-onset, and the severe proportion of GLP-1 RAs-associated gastrointestinal AEs were further analyzed.
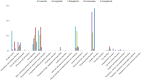
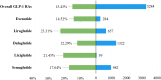
Results: A total of 21,281 reports of gastrointestinal toxicity were analyzed out of 81,752 adverse event reports, and the median age of the included patients was 62 (interquartile range [IQR] 54-70) years old. Overall GLP-1 RAs were associated with increased risk of gastrointestinal system disorders (ROR, 1.46; 95% CI, 1.44-1.49), which were further attributed to liraglutide (ROR, 2.39; 95% CI, 2.28-2.51), dulaglutide (ROR, 1.39; 95% CI, 1.36-1.42), and semaglutide (ROR, 3.00; 95% CI, 2.89-3.11). Adverse events uncovered in the labels included gastroesophageal reflux disease, gastritis, bezoar, breath odor, intra-abdominal hematoma, etc. Furthermore, it was observed that semaglutide had the greatest risk of nausea (ROR, 7.41; 95% CI, 7.10-7.74), diarrhea (ROR, 3.55; 95% CI, 3.35-3.77), vomiting (ROR, 6.67; 95% CI, 6.32-7.05), and constipation (ROR, 6.17; 95% CI, 5.72-6.66); liraglutide had the greatest risk of abdominal pain upper (ROR, 4.63; 95% CI, 4.12-5.21) and pancreatitis (ROR, 32.67; 95% CI, 29.44-36.25). Most gastrointestinal AEs tended to occur within one month. Liraglutide had the highest severe rate of gastrointestinal AEs (23.31%), while dulaglutide had the lowest, with a severe rate of 12.29%.
Conclusion: GLP-1 RA were significantly associated with gastrointestinal AEs, and the association was further attributed to liraglutide, dulaglutide, and semaglutide. In addition, semaglutide had the greatest risk of nausea, diarrhea, vomiting, constipation, and pancreatitis, while liraglutide had the greatest risk of upper abdominal pain. Our study provided valuable evidence for selecting appropriate GLP-1 RAs to avoid the occurrence of GLP-1 RA-induced gastrointestinal AEs.
Keywords: GLP-1 receptor agonists; adverse drug reactions; data mining; gastrointestinal toxicities; pharmacovigilance.
Copyright © 2022 Liu, Chen, Wang, Chen and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical