Gut microbiota and its derived SCFAs regulate the HPGA to reverse obesity-induced precocious puberty in female rats
- PMID: 36568086
- PMCID: PMC9782419
- DOI: 10.3389/fendo.2022.1051797
Gut microbiota and its derived SCFAs regulate the HPGA to reverse obesity-induced precocious puberty in female rats
Abstract
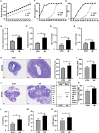
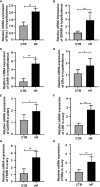
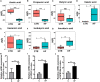
The intestinal microbiota and its derived short-chain fatty acids (SCFAs) can reverse obesity and obesity-related metabolic diseases, but whether it has an effect on obesity complicated by precocious puberty and its potential mechanism need to be further understood. The purpose of this study was to investigate the effect of the gut microbiota and its derived short-chain fatty acids (SCFAs) on obesity-induced precocious puberty rats and their regulatory mechanisms. We constructed obesity-induced precocious puberty rats using a high-fat diet (HFD) had notable similarity to precocious puberty caused by obesity due to overeating in children. We then added acetate, propionate, butyrate or their mixture to the HFD, and investigated the effect of intestinal microbiota and its derived SCFAs on the hypothalamic-pituitary-gonadal axis (HPGA) in rats with obesity-induced precocious puberty. We found that obesity-induced precocious puberty rats had an early first estrous cycle, increased hypothalamic mRNA expression of Kiss1, GPR54 and GnRH, and early gonadal maturation. Meanwhile, the intestinal microbiota imbalance and the main SCFAs production decreased in the colon. The addition of acetate, propionate, butyrate or their mixture to the HFD could significantly reverse the precocious puberty of rats, reduce GnRH release from the hypothalamus and delay the development of the gonadal axis through the Kiss1-GPR54-PKC-ERK1/2 pathway. Our findings suggest that gut microbiota-derived SCFAs are promising therapeutic means for the prevention of obesity-induced precocious puberty and provide new therapeutic strategies with clinical value.
Keywords: high-fat diet; microbiota; obesity; puberty; short chain fatty acids (SCFAs).
Copyright © 2022 Wang, Xu, Tan, Yi, Liu, Deng, Chen, Wang, Tian and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Comment in
-
Commentary: Gut microbiota and its derived SCFAs regulate the HPGA to reverse obesity-induced precocious puberty in female rats.Front Endocrinol (Lausanne). 2023 Jan 25;14:1121124. doi: 10.3389/fendo.2023.1121124. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36761186 Free PMC article. No abstract available.
Similar articles
-
Gut flora influences the hypothalamic-gonadal axis to regulate the pathogenesis of obesity-associated precocious puberty.Sci Rep. 2024 Nov 21;14(1):28844. doi: 10.1038/s41598-024-80140-8. Sci Rep. 2024. PMID: 39572735 Free PMC article.
-
Commentary: Gut microbiota and its derived SCFAs regulate the HPGA to reverse obesity-induced precocious puberty in female rats.Front Endocrinol (Lausanne). 2023 Jan 25;14:1121124. doi: 10.3389/fendo.2023.1121124. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36761186 Free PMC article. No abstract available.
-
Aspartame Intake Delayed Puberty Onset in Female Offspring Rats and Girls.Mol Nutr Food Res. 2024 Mar;68(5):e2300270. doi: 10.1002/mnfr.202300270. Epub 2024 Feb 22. Mol Nutr Food Res. 2024. PMID: 38389198
-
Anticancer Effects of Gut Microbiota-Derived Short-Chain Fatty Acids in Cancers.J Microbiol Biotechnol. 2023 Jul 28;33(7):849-856. doi: 10.4014/jmb.2301.01031. Epub 2023 Mar 30. J Microbiol Biotechnol. 2023. PMID: 37100764 Free PMC article. Review.
-
The impact of microbiota-derived short-chain fatty acids on macrophage activities in disease: Mechanisms and therapeutic potentials.Biomed Pharmacother. 2023 Sep;165:115276. doi: 10.1016/j.biopha.2023.115276. Epub 2023 Aug 4. Biomed Pharmacother. 2023. PMID: 37542852 Review.
Cited by
-
Alterations in the gut microbiota community are associated with childhood obesity and precocious puberty.BMC Microbiol. 2024 Aug 24;24(1):311. doi: 10.1186/s12866-024-03461-8. BMC Microbiol. 2024. PMID: 39182062 Free PMC article.
-
Causal relationship between the composition of the Gut Microbiota and central precocious puberty: a two-sample Mendelian randomization study.Front Pediatr. 2024 Nov 14;12:1438195. doi: 10.3389/fped.2024.1438195. eCollection 2024. Front Pediatr. 2024. PMID: 39624104 Free PMC article.
-
Exploring Oral and Vaginal Probiotic Solutions for Women's Health from Puberty to Menopause: A Narrative Review.Microorganisms. 2024 Aug 7;12(8):1614. doi: 10.3390/microorganisms12081614. Microorganisms. 2024. PMID: 39203456 Free PMC article. Review.
-
Relationship between high-fat diet, gut microbiota, and precocious puberty: mechanisms and implications.Front Microbiol. 2025 Jun 4;16:1564902. doi: 10.3389/fmicb.2025.1564902. eCollection 2025. Front Microbiol. 2025. PMID: 40535011 Free PMC article. Review.
-
Childhood obesity and central precocious puberty.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Jul 28;49(7):1034-1041. doi: 10.11817/j.issn.1672-7347.2024.240280. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39788491 Free PMC article. Review. Chinese, English.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

