Pasture intake protects against commercial diet-induced lipopolysaccharide production facilitated by gut microbiota through activating intestinal alkaline phosphatase enzyme in meat geese
- PMID: 36569878
- PMCID: PMC9774522
- DOI: 10.3389/fimmu.2022.1041070
Pasture intake protects against commercial diet-induced lipopolysaccharide production facilitated by gut microbiota through activating intestinal alkaline phosphatase enzyme in meat geese
Abstract
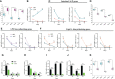
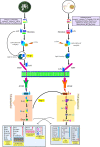
Introduction: Diet strongly affects gut microbiota composition, and gut bacteria can influence the intestinal barrier functions and systemic inflammation through metabolic endotoxemia. In-house feeding system (IHF, a low dietary fiber source) may cause altered cecal microbiota composition and inflammatory responses in meat geese via increased endotoxemia (lipopolysaccharides) with reduced intestinal alkaline phosphatase (ALP) production. The effects of artificial pasture grazing system (AGF, a high dietary fiber source) on modulating gut microbiota architecture and gut barrier functions have not been investigated in meat geese. Therefore, this study aimed to investigate whether intestinal ALP could play a critical role in attenuating reactive oxygen species (ROS) generation and ROS facilitating NF-κB pathway-induced systemic inflammation in meat geese.
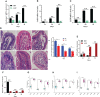
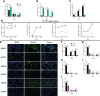
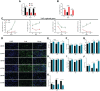
Methods: The impacts of IHF and AGF systems on gut microbial composition via 16 sRNA sequencing were assessed in meat geese. The host markers analysis through protein expression of serum and cecal tissues, hematoxylin and eosin (H&E) staining, localization of NF-қB and Nrf2 by immunofluorescence analysis, western blotting analysis of ALP, and quantitative PCR of cecal tissues was evaluated.
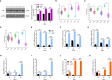
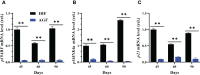
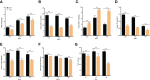
Results and discussion: In the gut microbiota analysis, meat geese supplemented with pasture showed a significant increase in commensal microbial richness and diversity compared to IHF meat geese demonstrating the antimicrobial, antioxidant, and anti-inflammatory ability of the AGF system. A significant increase in intestinal ALP-induced Nrf2 signaling pathway was confirmed representing LPS dephosphorylation mediated TLR4/MyD88 induced ROS reduction mechanisms in AGF meat geese. Further, the correlation analysis of top 44 host markers with gut microbiota showed that artificial pasture intake protected gut barrier functions via reducing ROS-mediated NF-κB pathway-induced gut permeability, systemic inflammation, and aging phenotypes. In conclusion, the intestinal ALP functions to regulate gut microbial homeostasis and barrier function appear to inhibit pro-inflammatory cytokines by reducing LPS-induced ROS production in AGF meat geese. The AGF system may represent a novel therapy to counteract the chronic inflammatory state leading to low dietary fiber-related diseases in animals.
Keywords: Keap1-Nrf2; gut microbiota; inflammation; intestinal alkaline phosphatase; lipopolysaccharides; meat geese; oxidative stress; pasture grazing.
Copyright © 2022 Ali, Ma, Farooq, Niu, Li, Li, Wang, Sun, Cui and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

