Sintilimab plus autologous NK cells as second-line treatment for advanced non-small-cell lung cancer previous treated with platinum-containing chemotherapy
- PMID: 36569881
- PMCID: PMC9773193
- DOI: 10.3389/fimmu.2022.1074906
Sintilimab plus autologous NK cells as second-line treatment for advanced non-small-cell lung cancer previous treated with platinum-containing chemotherapy
Abstract
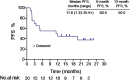
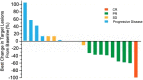
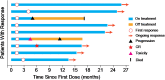
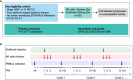
This pilot study (NCT03958097; https://www.clinicaltrials.gov/ct2/show/NCT03958097) was aimed to evaluate the efficacy and safety of PD-1 antibody combined autologous NK cells in the treatment of patients with stage IIIB/IIIC or IV non-small-cell lung cancer (NSCLC) who failed the first-line platinum-based chemotherapy. All patients received both sintilimab 200mg and 3×109 NK cells every 3 weeks. 20 patients were enrolled, median follow up time was 22.6 months. The median PFS was 11.6 months, ORR was 45%. Median OS was 17.7 months, 6-month OS rate and 12-month OS rate was 95.0% and 80.0%. Unexpected adverse events were not observed. 2 patients reported grade 3 adverse events (hypertriglyceridemia, neutropenia and increased creatine kinase). The autologous NK cells did not add extra adverse events to the ICI treatment. Autologous NK plus sintilimab showed promising antitumor activity and an acceptable safety profile in advanced driven-mutation negative NSCLC who failed on the first line treatment.
Keywords: NK cells; PD1 antibody; immune cell therapy; immunotherapy; non-small cell lung cancer.
Copyright © 2022 Jia, Chen, Chen, Niu, Liu, Ma, Wang, Yang, Zhao, Song, Lu, Chen, Cong, Wang, Xu, Cui, Liu, Chen, Li and Cui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

